当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Addition of Highly Polarized Organometallic Compounds to N-tert-Butanesulfinyl Imines in Deep Eutectic Solvents under Air: Preparation of Chiral Amines of Pharmaceutical Interest.
ChemSusChem ( IF 8.4 ) Pub Date : 2020-05-23 , DOI: 10.1002/cssc.202001142 Luciana Cicco 1 , Antonio Salomone 2 , Paola Vitale 1 , Nicolás Ríos-Lombardía 3 , Javier González-Sabín 3 , Joaquín García-Álvarez 4 , Filippo M Perna 1 , Vito Capriati 1, 5
ChemSusChem ( IF 8.4 ) Pub Date : 2020-05-23 , DOI: 10.1002/cssc.202001142 Luciana Cicco 1 , Antonio Salomone 2 , Paola Vitale 1 , Nicolás Ríos-Lombardía 3 , Javier González-Sabín 3 , Joaquín García-Álvarez 4 , Filippo M Perna 1 , Vito Capriati 1, 5
Affiliation
|
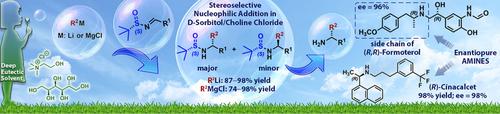
Highly polarized organometallic compounds of s‐block elements are added smoothly to chiral N‐tert‐butanesulfinyl imines in the biodegradable d ‐sorbitol/choline chloride eutectic mixture, thereby granting access to enantioenriched primary amines after quantitatively removing the sulfinyl group. The practicality of the method is further highlighted by proceeding at ambient temperature and under air, with very short reaction times (2 min), enabling the preparation of diastereoisomeric sulfinamides in very good yields (74–98 %) and with a broad substrate scope, and the possibility of scaling up the process. The method is demonstrated in the asymmetric syntheses of both the chiral amine side‐chain of (R ,R )‐Formoterol (96 % ee ) and the pharmaceutically relevant (R )‐Cinacalcet (98 % ee ).
中文翻译:

在空气下的深层共溶剂中,将高极性有机金属化合物加到N-叔丁亚磺酰基亚胺中:制备具有药用价值的手性胺。
将s嵌段元素的高度极化有机金属化合物平稳地添加到可生物降解的d-山梨糖醇/胆碱氯化物低共熔混合物中的手性N-叔丁烷亚磺酰基亚胺中,从而在定量去除亚硫酰基后可以使用对映体富集的伯胺。该方法的实用性在环境温度和空气中进行,反应时间非常短(2分钟),从而能够以非常高的收率(74–98%)制备非对映异构亚磺酰胺,而且底物范围广,从而进一步突出了该方法的实用性,以及扩大流程的可能性。(R,R)-福莫特罗的手性胺侧链的不对称合成证明了该方法(96% ee)和药学上相关的(R)-Cinacalcet(98% ee)。
更新日期:2020-07-22
中文翻译:

在空气下的深层共溶剂中,将高极性有机金属化合物加到N-叔丁亚磺酰基亚胺中:制备具有药用价值的手性胺。
将s嵌段元素的高度极化有机金属化合物平稳地添加到可生物降解的d-山梨糖醇/胆碱氯化物低共熔混合物中的手性N-叔丁烷亚磺酰基亚胺中,从而在定量去除亚硫酰基后可以使用对映体富集的伯胺。该方法的实用性在环境温度和空气中进行,反应时间非常短(2分钟),从而能够以非常高的收率(74–98%)制备非对映异构亚磺酰胺,而且底物范围广,从而进一步突出了该方法的实用性,以及扩大流程的可能性。(R,R)-福莫特罗的手性胺侧链的不对称合成证明了该方法(96% ee)和药学上相关的(R)-Cinacalcet(98% ee)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号