当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total synthesis of both enantiomers of clavigerins B and C
Tetrahedron ( IF 2.1 ) Pub Date : 2020-05-23 , DOI: 10.1016/j.tet.2020.131297 Nozomu Kakimoto , Yusuke Ogura , Hidenori Watanabe , Hirosato Takikawa
中文翻译:

锁骨蛋白B和C的两种对映体的全合成
更新日期:2020-05-23
Tetrahedron ( IF 2.1 ) Pub Date : 2020-05-23 , DOI: 10.1016/j.tet.2020.131297 Nozomu Kakimoto , Yusuke Ogura , Hidenori Watanabe , Hirosato Takikawa
|
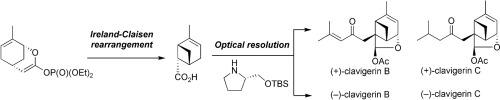
The first total synthesis of both enantiomers of clavigerins B and C, insect antifeedant sesquiterpenes isolated from New Zealand liverwort Lepidolaena clavigera, has been achieved. The synthesis features Ireland-Claisen rearrangement for construction of bicyclo[3.1.1]heptene skeleton. By comparison of the optical rotations of both enantiomers with those of natural clavigerins, the absolute configurations of natural clavigerins B and C were unambiguously determined to be 1S,3S,6S,8S,9R.
中文翻译:

锁骨蛋白B和C的两种对映体的全合成
分离自新西兰黑麦草鳞翅目的昆虫抗饲料倍半萜烯类的锁骨蛋白B和C的对映异构体的第一全合成已经实现。该合成具有爱尔兰-克莱森重排的特征,用于构建双环[3.1.1]庚烯骨架。通过与那些自然clavigerins的两种对映体的旋光的比较,自然clavigerins B和C的绝对构型明确地确定为1小号,3小号,6小号,8小号,9 - [R 。


























 京公网安备 11010802027423号
京公网安备 11010802027423号