Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2020-05-24 , DOI: 10.1016/j.apcata.2020.117646 Zhongxiao Yue , Manoj Pudukudy , Shiyu Chen , Yi Liu , Wenbo Zhao , Junya Wang , Shaoyun Shan , Qingming Jia
|
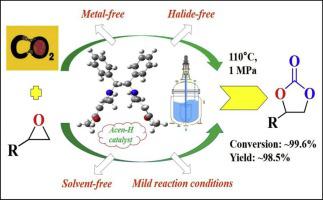
A metal-free Acen-H catalyst was effectively synthesized by a single step reflux method and successfully tested for the cycloaddition of CO2 with epoxides to synthesize cyclic carbonates. The Acen-H catalyst exhibited high activity and selectivity for the cycloaddition reaction in the absence of co-catalysts (halides) and solvents at mild reaction conditions of a reaction temperature of 110 °C and a reaction pressure of 1 MPa. Under the optimized reaction conditions, some of the epoxides were successfully converted into corresponding cyclic carbonates with a highest yield of ∼98.5%. The composition and structure of the homogeneous catalyst was then systematically evaluated and the reaction kinetics and a plausible reaction mechanism for the cycloaddition of CO2 with epoxides were proposed. The density functional theory (DFT) calculation provided a corroborated elucidation for the proposed mechanism. The catalytic activity of the Acen-H catalyst was found to be originated from the active hydrogen bond donors (-O-H, =N---H) and imino groups present (-N=) in it, which played a synergistic role in the adsorption and activation of reactants as confirmed by the DFT studies. The structural characteristics of the catalyst was found to be crucial for the cycloaddition of CO2 with epoxides.
中文翻译:

一种非金属Acen-H催化剂,用于在无溶剂和无卤化物的温和反应条件下将CO 2化学固定为环状碳酸酯
通过一步回流法有效地合成了无金属的Acen-H催化剂,并成功地测试了环氧化物对CO 2的环加成反应以合成环状碳酸酯。在反应温度为110℃,反应压力为1 MPa的温和反应条件下,在不存在助催化剂(卤化物)和溶剂的情况下,Acen-H催化剂对环加成反应表现出高活性和选择性。在优化的反应条件下,某些环氧化物成功地转化为相应的环状碳酸酯,最高收率为约98.5%。然后系统地评估了均相催化剂的组成和结构,以及反应动力学和合理的CO 2环加成反应机理。建议使用环氧化物。密度泛函理论(DFT)计算为所提出的机理提供了有力的解释。发现Acen-H催化剂的催化活性源自活性氢键供体(-OH,= N --- H)和其中存在的亚氨基(-N =),它们在催化作用中起着协同作用。 DFT研究证实了反应物的吸附和活化。发现该催化剂的结构特征对于CO 2与环氧化物的环加成至关重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号