当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Impact of pH and NaCl and CaCl2 Salts on the Speciation and Photochemistry of Pyruvic Acid in the Aqueous Phase.
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.jpca.0c01016 Man Luo 1 , Dorit Shemesh 2 , Michael N Sullivan 1 , Michael R Alves 1 , Meishi Song 1 , R Benny Gerber 2, 3 , Vicki H Grassian 1, 4
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.jpca.0c01016 Man Luo 1 , Dorit Shemesh 2 , Michael N Sullivan 1 , Michael R Alves 1 , Meishi Song 1 , R Benny Gerber 2, 3 , Vicki H Grassian 1, 4
Affiliation
|
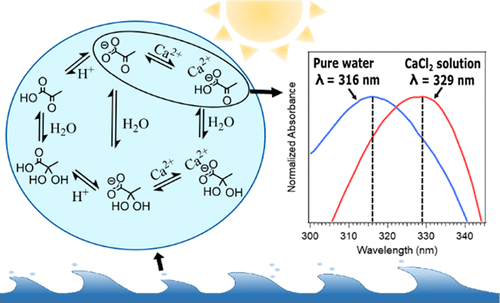
Recent studies have shown that pyruvic acid can produce higher-molecular-weight compounds upon irradiation in the aqueous phase. These compounds can contribute to the formation of secondary organic aerosols. There have been several previous studies on the effect of ionic strength on the photochemistry of pyruvic acid; however, few of them investigated the effects of marine relevant salts such as NaCl and CaCl2. In this study, we examine the effect of NaCl and CaCl2, namely, containing the coordinating cations Na+ and Ca2+, on the speciation, absorption properties, and photoreactivity of pyruvic acid in aqueous solutions of varying pH. NMR shows that both Ca2+ and Na+ further deprotonate pyruvic acid and decrease the diol to ketone ratio of pyruvic acid than in pure water at the same pH, especially at more acidic pH (pH less than 4). The absorption spectrum shows a strong red shift in the peak maxima for the n → π* transition of pyruvic acid in NaCl/CaCl2 solutions. This dependence is much more pronounced for divalent cations (Ca2+) compared to monovalent cations (Na+). Vertical excitation energy calculations of the anionic ketone form of pyruvic acid confirm the same red shift on the n → π* transition peak in the presence of Ca2+. In addition, the presence of NaCl/CaCl2 suppresses the photolysis rate of pyruvic acid, which could be due to the deprotonation of pyruvic acid by the cations and the lower photochemical reactivity for pyruvate, the deprotonated form.
中文翻译:

pH,NaCl和CaCl2盐对丙酮酸在水相中的形态和光化学的影响。
最近的研究表明,丙酮酸在水相中照射后可以产生更高分子量的化合物。这些化合物可有助于形成次级有机气溶胶。以前有一些关于离子强度对丙酮酸光化学影响的研究。但是,他们中很少有人研究海洋相关盐(如NaCl和CaCl 2)的影响。在这项研究中,我们研究了NaCl和CaCl 2(即含有配位阳离子Na +和Ca 2+)对丙酮酸在不同pH的水溶液中的形态,吸收性能和光反应性的影响。NMR显示Ca 2+和Na +与在相同pH值下,特别是在更酸性的pH值(pH小于4)下的纯水中相比,丙酮酸可以进一步使丙酮酸去质子化,并降低丙酮酸的二醇/酮比。吸收光谱在丙酮酸在NaCl / CaCl 2溶液中的n→π*跃迁处的峰最大值处显示出强烈的红移。与一价阳离子(Na +)相比,二价阳离子(Ca 2+)的依赖性更为明显。丙酮酸的阴离子酮形式的垂直激发能计算证实了在存在Ca 2+的情况下,n→π*跃迁峰上的红移相同。另外,NaCl / CaCl 2的存在 抑制丙酮酸的光解速率,这可能是由于丙酮酸被阳离子去质子化以及丙酮酸(去质子化形式)的光化学反应性降低。
更新日期:2020-06-25
中文翻译:

pH,NaCl和CaCl2盐对丙酮酸在水相中的形态和光化学的影响。
最近的研究表明,丙酮酸在水相中照射后可以产生更高分子量的化合物。这些化合物可有助于形成次级有机气溶胶。以前有一些关于离子强度对丙酮酸光化学影响的研究。但是,他们中很少有人研究海洋相关盐(如NaCl和CaCl 2)的影响。在这项研究中,我们研究了NaCl和CaCl 2(即含有配位阳离子Na +和Ca 2+)对丙酮酸在不同pH的水溶液中的形态,吸收性能和光反应性的影响。NMR显示Ca 2+和Na +与在相同pH值下,特别是在更酸性的pH值(pH小于4)下的纯水中相比,丙酮酸可以进一步使丙酮酸去质子化,并降低丙酮酸的二醇/酮比。吸收光谱在丙酮酸在NaCl / CaCl 2溶液中的n→π*跃迁处的峰最大值处显示出强烈的红移。与一价阳离子(Na +)相比,二价阳离子(Ca 2+)的依赖性更为明显。丙酮酸的阴离子酮形式的垂直激发能计算证实了在存在Ca 2+的情况下,n→π*跃迁峰上的红移相同。另外,NaCl / CaCl 2的存在 抑制丙酮酸的光解速率,这可能是由于丙酮酸被阳离子去质子化以及丙酮酸(去质子化形式)的光化学反应性降低。


























 京公网安备 11010802027423号
京公网安备 11010802027423号