当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Trifluoromethylthiolation, Trifluoromethylation, and Arylation Reactions of Difluoro Enol Silyl Ethers.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.joc.0c01030 Xingguo Jiang 1 , Denise Meyer 1 , Dominik Baran 1 , Miguel A Cortés González 1 , Kálmán J Szabó 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.joc.0c01030 Xingguo Jiang 1 , Denise Meyer 1 , Dominik Baran 1 , Miguel A Cortés González 1 , Kálmán J Szabó 1
Affiliation
|
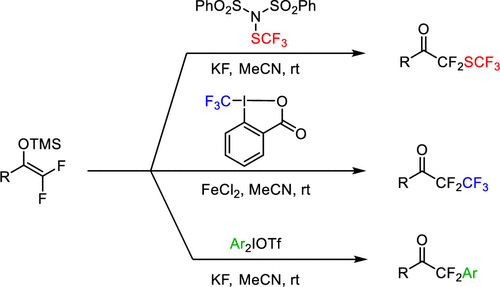
This study reports a new application area of difluoro enol silyl ethers, which can be easily obtained from trifluoromethyl ketones. The main focus has been directed to the electrophilic fluoroalkylation and arylation methods. The trifluoromethylthiolation of difluoro enol silyl ethers can be used for the construction of a novel trifluoromethylthio-α,α-difluoroketone (−COCF2SCF3) functionality. The −CF2SCF3 moiety has interesting properties due to the electron-withdrawing, albeit lipophilic, character of the SCF3 group, which can be combined with the high electrophilicity of the difluoroketone motif. The methodology could also be extended to difluoro homologation of the trifluoromethyl ketones using the Togni reagent. In addition, we presented a method for transition-metal-free arylation of difluoro enol silyl ethers based on hypervalent iodines.
中文翻译:

三氟甲基硫醇化,三氟甲基化和二氟烯醇甲硅烷基醚的芳基化反应。
这项研究报告了二氟烯醇甲硅烷基醚的一个新的应用领域,它可以很容易地从三氟甲基酮中获得。主要焦点已针对亲电性氟烷基化和芳基化方法。二氟烯醇甲硅烷基醚的三氟甲硫基化可用于构建新型三氟甲硫基-α,α-二氟酮(-COCF 2 SCF 3)官能团。的-CF 2 SCF 3部分具有由于SCF的吸电子,虽然亲脂性,字符有趣的性质3基团,其可以与二氟酮基序的高亲电性结合。该方法还可以扩展到使用Togni试剂对三氟甲基酮进行二氟同源化。此外,我们提出了一种基于高价碘的无氟二芳基甲硅烷基醚芳基化方法。
更新日期:2020-07-02
中文翻译:

三氟甲基硫醇化,三氟甲基化和二氟烯醇甲硅烷基醚的芳基化反应。
这项研究报告了二氟烯醇甲硅烷基醚的一个新的应用领域,它可以很容易地从三氟甲基酮中获得。主要焦点已针对亲电性氟烷基化和芳基化方法。二氟烯醇甲硅烷基醚的三氟甲硫基化可用于构建新型三氟甲硫基-α,α-二氟酮(-COCF 2 SCF 3)官能团。的-CF 2 SCF 3部分具有由于SCF的吸电子,虽然亲脂性,字符有趣的性质3基团,其可以与二氟酮基序的高亲电性结合。该方法还可以扩展到使用Togni试剂对三氟甲基酮进行二氟同源化。此外,我们提出了一种基于高价碘的无氟二芳基甲硅烷基醚芳基化方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号