当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights on Skin Sensitization to Linalool Hydroperoxides: EPR Evidence on Radical Intermediates Formation in Reconstructed Human Epidermis and 13C NMR Reactivity Studies with Thiol Residues.
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.chemrestox.0c00125 Salen Kuresepi 1 , Bertrand Vileno 2, 3 , Jean-Pierre Lepoittevin 1 , Elena Giménez-Arnau 1
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.chemrestox.0c00125 Salen Kuresepi 1 , Bertrand Vileno 2, 3 , Jean-Pierre Lepoittevin 1 , Elena Giménez-Arnau 1
Affiliation
|
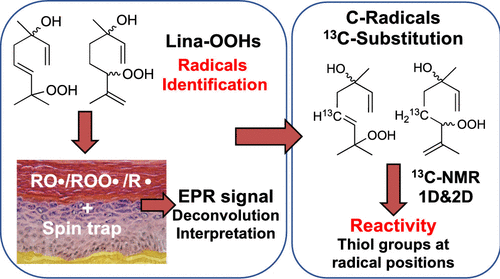
Linalool is one of the most commonly used fragrance terpenes in consumer products. While pure linalool is considered as non-allergenic because it has a very low skin sensitization potential, its autoxidation on air leads to allylic hydroperoxides that have been shown to be major skin sensitizers. These hydroperoxides have the potential to form antigens via radical mechanisms. In order to obtain in-depth insights of such reactivity, we first investigated the formation of free radicals derived from linalool hydroperoxides in situ in a model of human reconstructed epidermis by electron paramagnetic resonance combined with spin trapping. The formation of carbon- and oxygen-centered radical species derived from the hydroperoxides was especially evidenced in an epidermis model, mimicking human skin and thus closer to what may happen in vivo. To further investigate these results, we synthesized linalool hydroperoxides containing a 13C-substitution at positions precursor of carbon radicals to elucidate if one of these positions could react with cysteine, its thiol chemical function being one of the most labile groups prone to react through radical mechanisms. Reactions were followed by mono- and bidimensional 13C NMR. We validated that carbon radicals derived from allylic hydrogen abstraction by the initially formed alkoxyl radical and/or from its β-scission can alter directly the lateral chain of cysteine forming adducts via radical processes. Such results provide an original vision on the mechanisms likely involved in the reaction with thiol groups that might be present in the skin environment. Consequently, the present findings are a step ahead toward the understanding of protein binding processes to allergenic allylic hydroperoxides of linalool through the involvement of free radical species and thus of their sensitizing potential.
中文翻译:

敏化过氧化芳樟醇的皮肤的机械性见解:重组人表皮中自由基中间体形成的EPR证据和硫醇残基的13C NMR反应性研究。
芳樟醇是消费品中最常用的香萜。虽然纯的芳樟醇由于具有极低的皮肤致敏潜力而被认为是非过敏性的,但它在空气中的自氧化作用导致烯丙基氢过氧化物被证明是主要的皮肤致敏剂。这些氢过氧化物具有通过自由基机制形成抗原的潜力。为了获得对这种反应性的深入了解,我们首先研究了原位由芳樟醇过氧化氢衍生的自由基的形成电子顺磁共振结合自旋俘获的人类表皮模型。源自氢过氧化物的以碳和氧为中心的自由基物种的形成在表皮模型中得到了特别的证明,该模型模仿人类的皮肤,因此更接近于体内发生的事情。为了进一步研究这些结果,我们合成了在碳自由基前体位置含有13 C取代的芳樟醇氢过氧化物,以阐明这些位置之一是否可以与半胱氨酸反应,其硫醇化学功能是最易通过自由基反应的最不稳定的基团之一机制。反应之后是一维和二维131 H NMR。我们验证了通过最初形成的烷氧基从烯丙基氢提取和/或从其β断裂衍生的碳自由基可以通过自由基过程直接改变半胱氨酸形成加合物的侧链。这样的结果为皮肤环境中可能存在的与巯基反应的机理提供了初步的见解。因此,本发明的发现是通过自由基种类的参与及其对致敏潜能的理解,使蛋白质与芳樟醇的致敏烯丙基氢过氧化物的结合过程向前迈出了一步。
更新日期:2020-07-20
中文翻译:

敏化过氧化芳樟醇的皮肤的机械性见解:重组人表皮中自由基中间体形成的EPR证据和硫醇残基的13C NMR反应性研究。
芳樟醇是消费品中最常用的香萜。虽然纯的芳樟醇由于具有极低的皮肤致敏潜力而被认为是非过敏性的,但它在空气中的自氧化作用导致烯丙基氢过氧化物被证明是主要的皮肤致敏剂。这些氢过氧化物具有通过自由基机制形成抗原的潜力。为了获得对这种反应性的深入了解,我们首先研究了原位由芳樟醇过氧化氢衍生的自由基的形成电子顺磁共振结合自旋俘获的人类表皮模型。源自氢过氧化物的以碳和氧为中心的自由基物种的形成在表皮模型中得到了特别的证明,该模型模仿人类的皮肤,因此更接近于体内发生的事情。为了进一步研究这些结果,我们合成了在碳自由基前体位置含有13 C取代的芳樟醇氢过氧化物,以阐明这些位置之一是否可以与半胱氨酸反应,其硫醇化学功能是最易通过自由基反应的最不稳定的基团之一机制。反应之后是一维和二维131 H NMR。我们验证了通过最初形成的烷氧基从烯丙基氢提取和/或从其β断裂衍生的碳自由基可以通过自由基过程直接改变半胱氨酸形成加合物的侧链。这样的结果为皮肤环境中可能存在的与巯基反应的机理提供了初步的见解。因此,本发明的发现是通过自由基种类的参与及其对致敏潜能的理解,使蛋白质与芳樟醇的致敏烯丙基氢过氧化物的结合过程向前迈出了一步。


























 京公网安备 11010802027423号
京公网安备 11010802027423号