当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Preparation of Arenes with β-Stereogenic Centers: Confronting the 1,1-Disubstituted Olefin Problem Using CuH/Pd Cooperative Catalysis.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-05-21 , DOI: 10.1002/anie.202004414 Zhaohong Lu 1 , Stephen L Buchwald 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-05-21 , DOI: 10.1002/anie.202004414 Zhaohong Lu 1 , Stephen L Buchwald 1
Affiliation
|
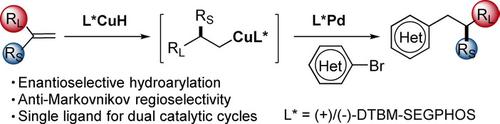
Arenes with β‐stereogenic centers are important substructures in pharmaceuticals and natural products. We have developed an asymmetric anti‐Markovnikov hydroarylation of 1,1‐disubstituted olefins by dual palladium and copper hydride catalysis as a convenient and general approach to access these substructures. This efficient one‐step process addresses several limitations of the traditional stepwise approaches. The use of cesium benzoate as a base and a common phosphine ligand for both the Cu‐ and Pd‐catalyzed processes were important discoveries that allow these challenging olefin substrates to be efficiently transformed. A variety of aryl bromide coupling partners, including numerous heterocycles, were coupled with 1,1‐disubstituted alkenes to generate arenes with β‐stereogenic centers.
中文翻译:

具有 β-立体中心的芳烃的对映选择性制备:使用 CuH/Pd 协同催化解决 1,1-二取代烯烃问题。
具有 β-立体中心的芳烃是药物和天然产物中的重要子结构。我们已经开发了一种通过钯和铜氢化物双催化的 1,1-二取代烯烃的不对称反马尔科夫尼科夫加氢芳基化反应,作为获得这些亚结构的一种方便和通用的方法。这种高效的一步法解决了传统逐步方法的几个局限性。在铜和钯催化过程中使用苯甲酸铯作为碱和常见的膦配体是重要的发现,可以有效地转化这些具有挑战性的烯烃底物。各种芳基溴偶联配偶体,包括许多杂环,与 1,1-二取代烯烃偶联生成具有 β-立体中心的芳烃。
更新日期:2020-05-21
中文翻译:

具有 β-立体中心的芳烃的对映选择性制备:使用 CuH/Pd 协同催化解决 1,1-二取代烯烃问题。
具有 β-立体中心的芳烃是药物和天然产物中的重要子结构。我们已经开发了一种通过钯和铜氢化物双催化的 1,1-二取代烯烃的不对称反马尔科夫尼科夫加氢芳基化反应,作为获得这些亚结构的一种方便和通用的方法。这种高效的一步法解决了传统逐步方法的几个局限性。在铜和钯催化过程中使用苯甲酸铯作为碱和常见的膦配体是重要的发现,可以有效地转化这些具有挑战性的烯烃底物。各种芳基溴偶联配偶体,包括许多杂环,与 1,1-二取代烯烃偶联生成具有 β-立体中心的芳烃。



























 京公网安备 11010802027423号
京公网安备 11010802027423号