Journal of Water Process Engineering ( IF 7 ) Pub Date : 2020-05-22 , DOI: 10.1016/j.jwpe.2020.101320 Ungwanen J. Ahile , Raymond A. Wuana , Adams U. Itodo , Rufus Sha’Ato , Renato F. Dantas
|
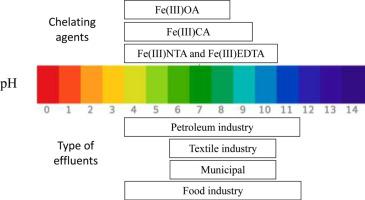
The stability of some iron-complexes was tested in photo-Fenton process performed at different conditions. Temperatures (298, 303, 308 and 313 K), acidic and alkaline pH and the HO attack in the complexes were investigated. Four iron chelates (CA, EDTA, NTA, OA) were used. The results revealed an increase in performance with increased temperature, both at acidic and neutral pH values. Studies on the stability of the iron chelates to pH show a reduced efficiency at higher pH with very poor performance of CA and OA at pH of 9. The effect of pH on attack of hydroxyl radicals on the chelate was also monitored using total iron reduction, the results show that the complexes are more stable at acidic than at alkaline pH, the suitability of the iron complexes for application at alkaline pH is in the order of EDTA > NTA > OA > Ca. The kinetic and thermodynamic parameters were also computed, judging from the values of coefficient of variation (r2), the contaminant decolorisation followed the pseudo-first-order kinetics at acidic pH, but followed the pseudo-second-order kinetics with the chelate assisted degradation at neutral pH. In each case, the rate constant increased with increasing temperature. The thermodynamic parameters show an endothermic and spontaneous degradation process which does not follow a complex mechanism.
attack in the complexes were investigated. Four iron chelates (CA, EDTA, NTA, OA) were used. The results revealed an increase in performance with increased temperature, both at acidic and neutral pH values. Studies on the stability of the iron chelates to pH show a reduced efficiency at higher pH with very poor performance of CA and OA at pH of 9. The effect of pH on attack of hydroxyl radicals on the chelate was also monitored using total iron reduction, the results show that the complexes are more stable at acidic than at alkaline pH, the suitability of the iron complexes for application at alkaline pH is in the order of EDTA > NTA > OA > Ca. The kinetic and thermodynamic parameters were also computed, judging from the values of coefficient of variation (r2), the contaminant decolorisation followed the pseudo-first-order kinetics at acidic pH, but followed the pseudo-second-order kinetics with the chelate assisted degradation at neutral pH. In each case, the rate constant increased with increasing temperature. The thermodynamic parameters show an endothermic and spontaneous degradation process which does not follow a complex mechanism.
中文翻译:

Fenton过程中铁螯合物的稳定性:pH,羟自由基攻击和温度的作用
在不同条件下通过光芬顿法测试了某些铁络合物的稳定性。温度(298、303、308和313 K),酸性和碱性pH值以及HO 配合物的攻击进行了调查。使用了四种铁螯合物(CA,EDTA,NTA,OA)。结果表明,在酸性和中性pH值下,性能均随温度升高而提高。铁螯合物对pH的稳定性研究表明,pH值为9时,在较高的pH值下效率降低,而CA和OA的性能非常差。还使用总铁还原量来监测pH值对螯合剂上羟基自由基攻击的影响,结果表明,该络合物在酸性条件下比在碱性pH条件下更稳定,铁配合物在碱性pH条件下的适用性依次为EDTA> NTA> OA>Ca。根据变异系数(r 2),污染物的脱色遵循在酸性pH下的拟一级动力学,但遵循在螯合剂辅助的中性pH下的降解的拟二级动力学。在每种情况下,速率常数都随温度升高而增加。热力学参数显示出吸热和自发降解过程,没有遵循复杂的机理。
配合物的攻击进行了调查。使用了四种铁螯合物(CA,EDTA,NTA,OA)。结果表明,在酸性和中性pH值下,性能均随温度升高而提高。铁螯合物对pH的稳定性研究表明,pH值为9时,在较高的pH值下效率降低,而CA和OA的性能非常差。还使用总铁还原量来监测pH值对螯合剂上羟基自由基攻击的影响,结果表明,该络合物在酸性条件下比在碱性pH条件下更稳定,铁配合物在碱性pH条件下的适用性依次为EDTA> NTA> OA>Ca。根据变异系数(r 2),污染物的脱色遵循在酸性pH下的拟一级动力学,但遵循在螯合剂辅助的中性pH下的降解的拟二级动力学。在每种情况下,速率常数都随温度升高而增加。热力学参数显示出吸热和自发降解过程,没有遵循复杂的机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号