Current Computer-Aided Drug Design ( IF 1.7 ) Pub Date : 2021-05-31 , DOI: 10.2174/1573409916666200520083215 Nam Q H Doan 1 , Tuyen N Truong 2 , Phuong T V Nguyen 2
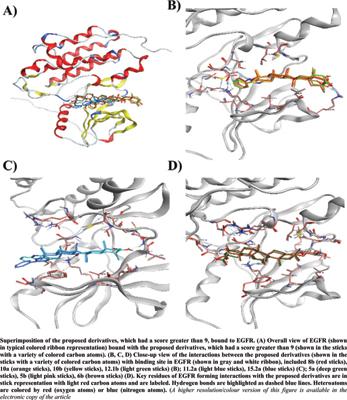
|
Background: In this study, the anti-colorectal cancer (CRC) activities of 40 glycyrrhetinic acid derivatives were proposed and evaluated by the molecular docking method, which allowed the flexibility of both ligand-receptor, with twelve CRC-related targets.
Methods: The proposed derivatives, which clearly distinguish isomers at position 18 as well as the different tautomers, were divided into five groups, including (1) glycyrrhetinic acid and its oxidation derivatives, (2) glycoside derivatives, (3) 3β-amine derivatives, (4) five-membered heterocyclic ring-combined derivatives, and (5) six-membered heterocyclic ring-combined derivatives.
Results: Finally, four out of twelve proposed targets related to CRC with good binding affinities to the proposed glycyrrhetinic acid derivatives were selected, including Epidermal Growth Factor Receptor (EGFR), Focal Adhesion Kinase (FAK), Lactate Dehydrogenase A (LDHA), and Thymidylate Synthase (TS).
Conclusion: From there, 9/40 derivatives for EGFR (pKd ≥ 9); 10/40 derivatives for FAK (pKd ≥ 10); 9/40 derivatives for LDHA (pKd ≥ 10), and 6/40 derivatives for TS (pKd ≥ 9) were also obtained. The glycoside derivatives showed the best binding affinity (especially the glucuronide derivative 5b), followed by the 3β-amino derivatives (especially the 3β-(phenylamino) derivative 8b) and the five-membered heterocyclic ring-combined derivatives (especially the pyrrole derivative 10a or pyrazole derivative 11.2a), while the six-membered heterocyclic ring-combined derivatives had less potential to inhibit the 4 selected targets.
中文翻译:

甘草次酸衍生物抗结直肠癌药物的分子对接研究
背景:在本研究中,通过分子对接方法提出并评估了 40 种甘草次酸衍生物的抗结直肠癌 (CRC) 活性,该方法允许配体 - 受体具有灵活性,具有 12 个 CRC 相关靶点。
方法:将18位异构体和不同互变异构体明确区分的拟议衍生物分为五组,包括(1)甘草次酸及其氧化衍生物,(2)糖苷衍生物,(3)3β-胺衍生物,(4)五元杂环组合衍生物,和(5)六元杂环组合衍生物。
结果:最后,选择了与 CRC 相关的 12 个与所提议的甘草次酸衍生物具有良好结合亲和力的 4 个目标,包括表皮生长因子受体 (EGFR)、粘着斑激酶 (FAK)、乳酸脱氢酶 A (LDHA) 和胸苷酸合酶 (TS)。
结论:从那里,EGFR 的 9/40 衍生物(pK d ≥ 9);FAK 的 10/40 衍生物(pK d ≥ 10);还获得了 LDHA 的 9/40 衍生物(pK d ≥ 10)和 TS 的 6/40 衍生物(pK d ≥ 9)。糖苷衍生物表现出最好的结合亲和力(尤其是葡萄糖醛酸衍生物5b),其次是3β-氨基衍生物(尤其是3β-(苯氨基)衍生物8b)和五元杂环组合衍生物(尤其是吡咯衍生物10a)或吡唑衍生物 11.2a),而六元杂环组合衍生物抑制 4 个选定目标的潜力较小。


























 京公网安备 11010802027423号
京公网安备 11010802027423号