当前位置:
X-MOL 学术
›
Photochem. Photobiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Luminescence Activity Decreases When v ‐Coelenterazine Replaces Coelenterazine in Calcium‐regulated Photoprotein – A Theoretical and Experimental Study
Photochemistry and Photobiology ( IF 3.3 ) Pub Date : 2020-06-24 , DOI: 10.1111/php.13280 Bo-Wen Ding 1 , Elena V Eremeeva 2 , Eugene S Vysotski 2 , Ya-Jun Liu 1
Photochemistry and Photobiology ( IF 3.3 ) Pub Date : 2020-06-24 , DOI: 10.1111/php.13280 Bo-Wen Ding 1 , Elena V Eremeeva 2 , Eugene S Vysotski 2 , Ya-Jun Liu 1
Affiliation
|
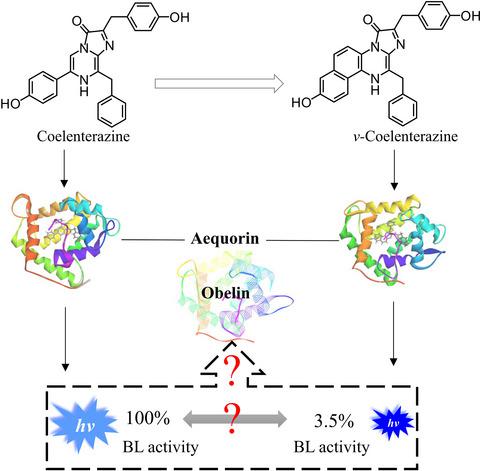
Calcium‐regulated photoproteins are found in at least five phyla of organisms. The light emitted by those photoproteins can be tuned by mutating the photoprotein and/or by modifying the substrate coelenterazine (CTZ). Thirty years ago, Shimomura observed that the luminescence activity of aequorin was dramatically reduced when the substrate CTZ was replaced by its analog v‐CTZ. The latter is formed by adding a phenyl ring to the π‐conjugated moiety of CTZ. The decrease in luminescence activity has not been understood until now. In this paper, through combined quantum mechanics and molecular mechanics calculations as well as molecular dynamics simulations, we discovered the reason for this observation. Modification of the substrate changes the conformation of nearby aromatic residues and enhances the π‐π stacking interactions between the conjugated moiety of v‐CTZ and the residues, which weakens the charge transfer to form light emitter and leads to a lower luminescence activity. The microenvironments of CTZ in obelin and in aequorin are very similar, so we predicted that the luminescence activity of obelin will also dramatically decrease when CTZ is replaced by v‐CTZ. This prediction has received strong evidence from currently theoretical calculations and has been verified by experiments.
中文翻译:

当 v-腔肠素替代钙调节光蛋白中的腔肠素时,发光活性降低——理论和实验研究
在至少五个生物门中发现了钙调节的光蛋白。可以通过改变发光蛋白和/或修饰底物腔肠素 (CTZ) 来调整这些发光蛋白发出的光。三十年前,Shimomura 观察到当底物 CTZ 被其类似物 v-CTZ 取代时,水母发光蛋白的发光活性显着降低。后者是通过将苯环添加到 CTZ 的 π 共轭部分形成的。直到现在才了解发光活性的降低。在本文中,通过结合量子力学和分子力学计算以及分子动力学模拟,我们发现了这一观察结果的原因。底物的修饰改变了附近芳香残基的构象,并增强了 v-CTZ 的共轭部分与残基之间的 π-π 堆积相互作用,这削弱了形成发光体的电荷转移并导致较低的发光活性。CTZ在obelin和水母发光蛋白中的微环境非常相似,因此我们预测当CTZ被v-CTZ取代时,obelin的发光活性也会急剧下降。这一预测已经从目前的理论计算中得到了强有力的证据,并得到了实验的验证。因此我们预测当 CTZ 被 v-CTZ 取代时,obelin 的发光活性也会急剧下降。这一预测已经从目前的理论计算中得到了强有力的证据,并得到了实验的验证。因此我们预测当 CTZ 被 v-CTZ 取代时,obelin 的发光活性也会急剧下降。这一预测已经从目前的理论计算中得到了强有力的证据,并得到了实验的验证。
更新日期:2020-06-24
中文翻译:

当 v-腔肠素替代钙调节光蛋白中的腔肠素时,发光活性降低——理论和实验研究
在至少五个生物门中发现了钙调节的光蛋白。可以通过改变发光蛋白和/或修饰底物腔肠素 (CTZ) 来调整这些发光蛋白发出的光。三十年前,Shimomura 观察到当底物 CTZ 被其类似物 v-CTZ 取代时,水母发光蛋白的发光活性显着降低。后者是通过将苯环添加到 CTZ 的 π 共轭部分形成的。直到现在才了解发光活性的降低。在本文中,通过结合量子力学和分子力学计算以及分子动力学模拟,我们发现了这一观察结果的原因。底物的修饰改变了附近芳香残基的构象,并增强了 v-CTZ 的共轭部分与残基之间的 π-π 堆积相互作用,这削弱了形成发光体的电荷转移并导致较低的发光活性。CTZ在obelin和水母发光蛋白中的微环境非常相似,因此我们预测当CTZ被v-CTZ取代时,obelin的发光活性也会急剧下降。这一预测已经从目前的理论计算中得到了强有力的证据,并得到了实验的验证。因此我们预测当 CTZ 被 v-CTZ 取代时,obelin 的发光活性也会急剧下降。这一预测已经从目前的理论计算中得到了强有力的证据,并得到了实验的验证。因此我们预测当 CTZ 被 v-CTZ 取代时,obelin 的发光活性也会急剧下降。这一预测已经从目前的理论计算中得到了强有力的证据,并得到了实验的验证。


























 京公网安备 11010802027423号
京公网安备 11010802027423号