当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Promiscuous activity of 3‐isopropylmalate dehydrogenase produced at physiological level affords Escherichia coli growth on D‐malate
FEBS Letters ( IF 3.5 ) Pub Date : 2020-06-02 , DOI: 10.1002/1873-3468.13814 Mohammad Shahneawz Khan 1, 2 , Serena Gargiulo 1 , Patrice Soumillion 1
FEBS Letters ( IF 3.5 ) Pub Date : 2020-06-02 , DOI: 10.1002/1873-3468.13814 Mohammad Shahneawz Khan 1, 2 , Serena Gargiulo 1 , Patrice Soumillion 1
Affiliation
|
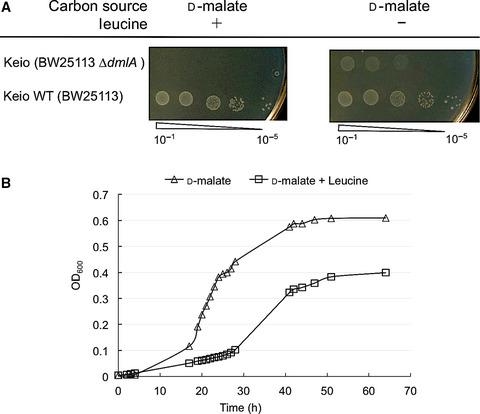
Promiscuous activities of enzymes may serve as starting points for the evolution of new functions. However, most experimental examples of promiscuity affording an observable phenotype necessitate the artificial overexpression of the target enzyme. Here, we show that 3-isopropylmalate dehydrogenase (IPMDH), an enzyme involved in leucine biosynthesis, has a secondary activity on D-malate, which is sufficient for D-malate assimilation under physiological conditions where the enzyme is upregulated. In vitro, the turnover constant (kcat ) of IPMDH for D-malate is about 30-fold lower than the kcat for 3-isopropylmalate, yet sufficiently high to support the growth on D-malate. From an evolutionary perspective, our results highlight the possibility of phenotype emergence triggered by arbitrary changes in environmental conditions and prior to any mutational event.
中文翻译:

在生理水平产生的 3-异丙基苹果酸脱氢酶的混杂活性使大肠杆菌在 D-苹果酸上生长
酶的混杂活动可以作为新功能进化的起点。然而,大多数提供可观察表型的滥交实验实例都需要目标酶的人工过度表达。在这里,我们展示了 3-异丙基苹果酸脱氢酶 (IPMDH),一种参与亮氨酸生物合成的酶,对 D-苹果酸具有二级活性,这足以在酶上调的生理条件下同化 D-苹果酸。在体外,IPMDH 对 D-苹果酸的周转常数 (kcat) 比对 3-异丙基苹果酸的 kcat 低约 30 倍,但足够高以支持 D-苹果酸的生长。从进化的角度来看,
更新日期:2020-06-02
中文翻译:

在生理水平产生的 3-异丙基苹果酸脱氢酶的混杂活性使大肠杆菌在 D-苹果酸上生长
酶的混杂活动可以作为新功能进化的起点。然而,大多数提供可观察表型的滥交实验实例都需要目标酶的人工过度表达。在这里,我们展示了 3-异丙基苹果酸脱氢酶 (IPMDH),一种参与亮氨酸生物合成的酶,对 D-苹果酸具有二级活性,这足以在酶上调的生理条件下同化 D-苹果酸。在体外,IPMDH 对 D-苹果酸的周转常数 (kcat) 比对 3-异丙基苹果酸的 kcat 低约 30 倍,但足够高以支持 D-苹果酸的生长。从进化的角度来看,



























 京公网安备 11010802027423号
京公网安备 11010802027423号