当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Calcium triggers the dissociation of Myosin Va from ribosomes in ribonucleoprotein complexes
FEBS Letters ( IF 3.5 ) Pub Date : 2020-05-30 , DOI: 10.1002/1873-3468.13813 Lucía Canclini 1 , Karina Cal 2 , Camila Bardier 1 , Paul Ruiz 2 , John A Mercer 3 , Aldo Calliari 2
FEBS Letters ( IF 3.5 ) Pub Date : 2020-05-30 , DOI: 10.1002/1873-3468.13813 Lucía Canclini 1 , Karina Cal 2 , Camila Bardier 1 , Paul Ruiz 2 , John A Mercer 3 , Aldo Calliari 2
Affiliation
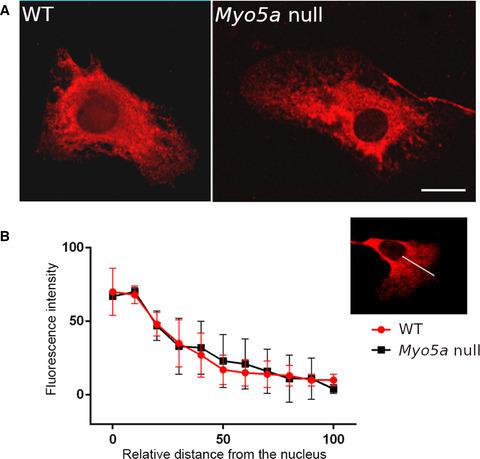
|
The sorting of RNAs to specific regions of the cell for local translation represents an important mechanism directing protein distribution and cell compartmentalization. While significant progress has been made in understanding the mechanisms underlying the transport and localization of mRNAs, the mechanisms governing ribosome mobilization are less well understood. Ribosomes present in the cytoplasm of multiple cell types can form ribonucleoprotein complexes that also contain myosin‐Va (Myo5a), a processive, actin‐dependent molecular motor. Here, we report that Myo5a can be disassociated from ribosomes when ribonucleoprotein complexes are exposed to calcium, both in vitro and in vivo. We suggest that Myo5a may act as a molecular switch able to anchor or release ribosomes from the actin cytoskeleton in response to intracellular signaling.
中文翻译:

钙触发肌球蛋白 Va 从核糖核蛋白复合物中的核糖体解离
将 RNA 分类到细胞的特定区域以进行局部翻译代表了指导蛋白质分布和细胞区室化的重要机制。虽然在理解 mRNA 运输和定位的机制方面取得了重大进展,但对核糖体动员的机制了解较少。存在于多种细胞类型的细胞质中的核糖体可以形成核糖核蛋白复合物,该复合物还包含肌球蛋白-Va (Myo5a),这是一种进行性的、肌动蛋白依赖性分子马达。在这里,我们报告了当核糖核蛋白复合物在体外和体内暴露于钙时,Myo5a 可以与核糖体分离。我们认为,Myo5a 可能作为一种分子开关,能够响应细胞内信号转导从肌动蛋白细胞骨架锚定或释放核糖体。
更新日期:2020-05-30
中文翻译:

钙触发肌球蛋白 Va 从核糖核蛋白复合物中的核糖体解离
将 RNA 分类到细胞的特定区域以进行局部翻译代表了指导蛋白质分布和细胞区室化的重要机制。虽然在理解 mRNA 运输和定位的机制方面取得了重大进展,但对核糖体动员的机制了解较少。存在于多种细胞类型的细胞质中的核糖体可以形成核糖核蛋白复合物,该复合物还包含肌球蛋白-Va (Myo5a),这是一种进行性的、肌动蛋白依赖性分子马达。在这里,我们报告了当核糖核蛋白复合物在体外和体内暴露于钙时,Myo5a 可以与核糖体分离。我们认为,Myo5a 可能作为一种分子开关,能够响应细胞内信号转导从肌动蛋白细胞骨架锚定或释放核糖体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号