当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
RecJ from Bacillus halodurans possesses endonuclease activity at moderate temperature
FEBS Letters ( IF 3.5 ) Pub Date : 2020-06-15 , DOI: 10.1002/1873-3468.13809 Wen Wang 1, 2 , Liya Ma 1, 2 , Ling Wang 1 , Li Zheng 2 , Minggang Zheng 2
FEBS Letters ( IF 3.5 ) Pub Date : 2020-06-15 , DOI: 10.1002/1873-3468.13809 Wen Wang 1, 2 , Liya Ma 1, 2 , Ling Wang 1 , Li Zheng 2 , Minggang Zheng 2
Affiliation
|
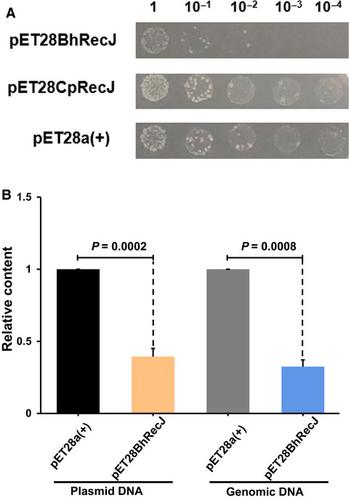
RecJ homologs, which occur in virtually all prokaryotes and eukaryotes, play key roles in DNA damage repair and recombination. Current evidence shows that RecJ family proteins exhibit exonuclease activity, degrading single‐stranded nucleic acids. Here, we report a novel RecJ isolated from Bacillus halodurans, which utilizes double‐stranded DNA as a substrate and functions as an endonuclease. Bacillus halodurans RecJ (BhRecJ) cleaves supercoiled plasmids into open circular and linear forms. Besides the typical domains of DHH, DHHA1, and oligonucleotide‐binding‐fold, BhRecJ possesses a C‐terminal domain with unknown function, which might form the core of the endonuclease activity. Using mutational analysis, we mapped several essential residues for BhRecJ endonuclease activity. Our findings suggest that BhRecJ may be involved in biological processes not typically associated with RecJ proteins.
中文翻译:

来自嗜盐芽孢杆菌的 RecJ 在中等温度下具有核酸内切酶活性
RecJ 同源物几乎存在于所有原核生物和真核生物中,在 DNA 损伤修复和重组中发挥关键作用。目前的证据表明 RecJ 家族蛋白表现出外切核酸酶活性,降解单链核酸。在这里,我们报告了一种从嗜盐芽孢杆菌中分离的新型 RecJ,它利用双链 DNA 作为底物并作为核酸内切酶发挥作用。耐盐芽孢杆菌 RecJ (BhRecJ) 将超螺旋质粒切割成开环和线性形式。除了 DHH、DHHA1 和寡核苷酸结合折叠的典型结构域外,BhRecJ 还具有一个功能未知的 C 端结构域,它可能构成核酸内切酶活性的核心。使用突变分析,我们绘制了 BhRecJ 核酸内切酶活性的几个必需残基。
更新日期:2020-06-15
中文翻译:

来自嗜盐芽孢杆菌的 RecJ 在中等温度下具有核酸内切酶活性
RecJ 同源物几乎存在于所有原核生物和真核生物中,在 DNA 损伤修复和重组中发挥关键作用。目前的证据表明 RecJ 家族蛋白表现出外切核酸酶活性,降解单链核酸。在这里,我们报告了一种从嗜盐芽孢杆菌中分离的新型 RecJ,它利用双链 DNA 作为底物并作为核酸内切酶发挥作用。耐盐芽孢杆菌 RecJ (BhRecJ) 将超螺旋质粒切割成开环和线性形式。除了 DHH、DHHA1 和寡核苷酸结合折叠的典型结构域外,BhRecJ 还具有一个功能未知的 C 端结构域,它可能构成核酸内切酶活性的核心。使用突变分析,我们绘制了 BhRecJ 核酸内切酶活性的几个必需残基。

























 京公网安备 11010802027423号
京公网安备 11010802027423号