当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal structures of the sheeppoxvirus encoded inhibitor of apoptosis SPPV14 bound to Hrk and Bax BH3 peptides
FEBS Letters ( IF 3.5 ) Pub Date : 2020-05-30 , DOI: 10.1002/1873-3468.13807 Chathura D Suraweera 1 , Denis R Burton 1 , Mark G Hinds 2 , Marc Kvansakul 1
FEBS Letters ( IF 3.5 ) Pub Date : 2020-05-30 , DOI: 10.1002/1873-3468.13807 Chathura D Suraweera 1 , Denis R Burton 1 , Mark G Hinds 2 , Marc Kvansakul 1
Affiliation
|
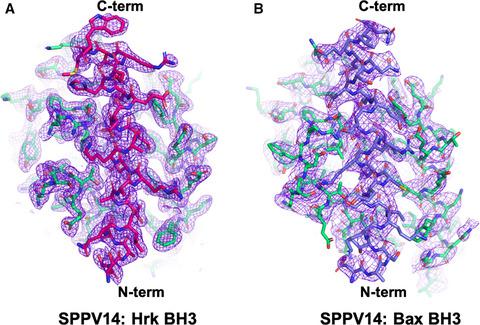
Programmed death of infected cells is used by multicellular organisms to counter viral infections. Sheeppox virus encodes for SPPV14, a potent inhibitor of Bcl‐2‐mediated apoptosis. We reveal the structural basis of apoptosis inhibition by determining crystal structures of SPPV14 bound to BH3 motifs of proapoptotic Bax and Hrk. The structures show that SPPV14 engages BH3 peptides using the canonical ligand‐binding groove. Unexpectedly, Arg84 from SPPV14 forms an ionic interaction with the conserved Asp in the BH3 motif in a manner that replaces the canonical ionic interaction seen in almost all host Bcl‐2:BH3 motif complexes. These results reveal the flexibility of virus‐encoded Bcl‐2 proteins to mimic key interactions from endogenous host signalling pathways to retain BH3 binding and prosurvival functionality.
中文翻译:

与 Hrk 和 Bax BH3 肽结合的羊痘病毒编码的凋亡抑制剂 SPPV14 的晶体结构
受感染细胞的程序性死亡被多细胞生物用来对抗病毒感染。羊痘病毒编码 SPPV14,一种有效的 Bcl-2 介导的细胞凋亡抑制剂。我们通过确定与促凋亡 Bax 和 Hrk 的 BH3 基序结合的 SPPV14 的晶体结构来揭示细胞凋亡抑制的结构基础。结构表明 SPPV14 使用经典配体结合槽与 BH3 肽结合。出乎意料的是,来自 SPPV14 的 Arg84 与 BH3 基序中保守的 Asp 形成离子相互作用,这种相互作用取代了几乎所有宿主 Bcl-2:BH3 基序复合物中的典型离子相互作用。这些结果揭示了病毒编码的 Bcl-2 蛋白在模拟来自内源性宿主信号通路的关键相互作用以保留 BH3 结合和促存活功能方面的灵活性。
更新日期:2020-05-30
中文翻译:

与 Hrk 和 Bax BH3 肽结合的羊痘病毒编码的凋亡抑制剂 SPPV14 的晶体结构
受感染细胞的程序性死亡被多细胞生物用来对抗病毒感染。羊痘病毒编码 SPPV14,一种有效的 Bcl-2 介导的细胞凋亡抑制剂。我们通过确定与促凋亡 Bax 和 Hrk 的 BH3 基序结合的 SPPV14 的晶体结构来揭示细胞凋亡抑制的结构基础。结构表明 SPPV14 使用经典配体结合槽与 BH3 肽结合。出乎意料的是,来自 SPPV14 的 Arg84 与 BH3 基序中保守的 Asp 形成离子相互作用,这种相互作用取代了几乎所有宿主 Bcl-2:BH3 基序复合物中的典型离子相互作用。这些结果揭示了病毒编码的 Bcl-2 蛋白在模拟来自内源性宿主信号通路的关键相互作用以保留 BH3 结合和促存活功能方面的灵活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号