当前位置:
X-MOL 学术
›
Biopolymers
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrogen sulfide concentration in the milieu of the hydrated alanine dipeptide determines its polyproline II‐beta propensity: Main chain contribution to the energetic origin of the formation of amyloid
Biopolymers ( IF 2.9 ) Pub Date : 2020-05-07 , DOI: 10.1002/bip.23356 Noemi G Mirkin 1 , Samuel Krimm 1
Biopolymers ( IF 2.9 ) Pub Date : 2020-05-07 , DOI: 10.1002/bip.23356 Noemi G Mirkin 1 , Samuel Krimm 1
Affiliation
|
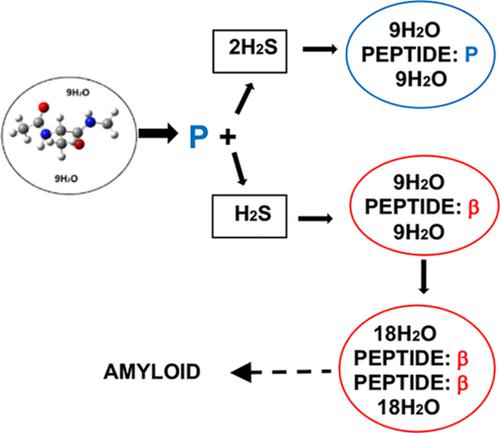
In view of the observation that the concentration of hydrogen sulfide in brains with Alzheimer's disease (AD) is lower than that in normal brains and in line with our previous studies indicating that additional content in the aqueous environment (milieu) of a peptide can change its local energetic preference from a polyproline II (P) to a β conformation (and therefore its tendency to form the β-chain structures that lead to the amyloid plaques associated with the disease), we have studied the effect of H2 S concentration on such propensity in a simple model peptide, the alanine dipeptide (ADP). The two concentration states are represented by ADP(H2 O)18 (H2 S) and ADP(H2 O)18 (H2 S)2 . Ab initio calculations of these structures show that the lowest energy of the former is a β conformation while that of the latter is a P, mirroring the observed AD results and strengthening our proposal that amyloid diseases are better viewed in the context of a protein milieu-folding paradigm.
中文翻译:

水合丙氨酸二肽环境中的硫化氢浓度决定了其聚脯氨酸 II-β 倾向:主链对淀粉样蛋白形成的能量起源的贡献
鉴于观察到患有阿尔茨海默病 (AD) 的大脑中硫化氢的浓度低于正常大脑中的浓度,并且与我们之前的研究表明肽的水性环境(环境)中的额外含量可以改变其局部能量偏好从聚脯氨酸 II (P) 到 β 构象(因此它倾向于形成导致与疾病相关的淀粉样斑块的 β 链结构),我们研究了 H2 S 浓度对这种倾向的影响在一个简单的模型肽中,丙氨酸二肽 (ADP)。这两种浓度状态由 ADP(H2 O)18 (H2 S) 和 ADP(H2 O)18 (H2 S)2 表示。这些结构的从头算计算表明,前者的最低能量是 β 构象,而后者的最低能量是 P,
更新日期:2020-05-07
中文翻译:

水合丙氨酸二肽环境中的硫化氢浓度决定了其聚脯氨酸 II-β 倾向:主链对淀粉样蛋白形成的能量起源的贡献
鉴于观察到患有阿尔茨海默病 (AD) 的大脑中硫化氢的浓度低于正常大脑中的浓度,并且与我们之前的研究表明肽的水性环境(环境)中的额外含量可以改变其局部能量偏好从聚脯氨酸 II (P) 到 β 构象(因此它倾向于形成导致与疾病相关的淀粉样斑块的 β 链结构),我们研究了 H2 S 浓度对这种倾向的影响在一个简单的模型肽中,丙氨酸二肽 (ADP)。这两种浓度状态由 ADP(H2 O)18 (H2 S) 和 ADP(H2 O)18 (H2 S)2 表示。这些结构的从头算计算表明,前者的最低能量是 β 构象,而后者的最低能量是 P,


























 京公网安备 11010802027423号
京公网安备 11010802027423号