当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrophilic Cyanative Alkenylation of Arenes.
Organic Letters ( IF 5.2 ) Pub Date : 2020-05-20 , DOI: 10.1021/acs.orglett.0c01204 Mingyue Zhao 1 , Alejandro G Barrado 1 , Kristin Sprenger 1 , Christopher Golz 1 , Ricardo A Mata 2 , Manuel Alcarazo 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-05-20 , DOI: 10.1021/acs.orglett.0c01204 Mingyue Zhao 1 , Alejandro G Barrado 1 , Kristin Sprenger 1 , Christopher Golz 1 , Ricardo A Mata 2 , Manuel Alcarazo 1
Affiliation
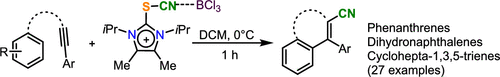
|
A variety of appropriately substituted internal alkynes were transformed into the corresponding cyano-substituted phenanthrenes, dihydronaphthalenes, and cyclohepta-1,3,5-trienes in moderate to excellent yields by treatment with imidazolium thiocyanate 1, which serves as an easy to handle [CN]+ precursor, in the presence of BCl3. The synthetic value of the method is additionally demonstrated by the transformation of the primarily obtained products into heavily substituted quinolines. Additionally, the dynamic properties of the prepared dibenzocyclohepta-1,3,5-trienes have been investigated.
中文翻译:

芳烃的亲电氰基烯化。
通过使用易处理的硫氰酸咪唑鎓1,将各种适当取代的内部炔烃以中等至极好的收率转化为相应的氰基取代的菲,二氢萘和环庚-1,3,5-三烯。] +前体,在BCl 3存在下。该方法的综合价值还通过将最初获得的产物转化为高度取代的喹啉而得到证明。另外,还研究了制备的二苯并环庚-1,3,5-三烯的动力学性质。
更新日期:2020-07-02
中文翻译:

芳烃的亲电氰基烯化。
通过使用易处理的硫氰酸咪唑鎓1,将各种适当取代的内部炔烃以中等至极好的收率转化为相应的氰基取代的菲,二氢萘和环庚-1,3,5-三烯。] +前体,在BCl 3存在下。该方法的综合价值还通过将最初获得的产物转化为高度取代的喹啉而得到证明。另外,还研究了制备的二苯并环庚-1,3,5-三烯的动力学性质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号