The Plant Cell ( IF 11.6 ) Pub Date : 2020-08-01 , DOI: 10.1105/tpc.19.00752 Aashima Khosla 1 , Nicholas Morffy 2 , Qingtian Li 1 , Lionel Faure 2, 3 , Sun Hyun Chang 1 , Jiaren Yao 4, 5 , Jiameng Zheng 1 , Mei L Cai 1 , John Stanga 2, 6 , Gavin R Flematti 4 , Mark T Waters 4, 5 , David C Nelson 7
|
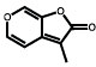
Karrikins (KARs) are butenolides found in smoke that can influence germination and seedling development of many plants. The KAR signaling mechanism is hypothesized to be very similar to that of the plant hormone strigolactone (SL). Both pathways require the F-box protein MORE AXILLARY GROWTH2 (MAX2), and other core signaling components have shared ancestry. Putatively, KAR activates the receptor KARRIKIN INSENSITIVE2 (KAI2), triggering its association with the E3 ubiquitin ligase complex SCFMAX2 and downstream targets SUPPRESSOR OF MAX2 1 (SMAX1) and SMAX1-LIKE2 (SMXL2). Polyubiquitination and proteolysis of SMAX1 and SMXL2 then enable growth responses to KAR. However, many of the assumptions of this model have not been demonstrated. Therefore, we investigated the posttranslational regulation of SMAX1 from the model plant Arabidopsis (Arabidopsis thaliana). We find evidence that SMAX1 is degraded by KAI2-SCFMAX2 but is also subject to MAX2-independent turnover. We identify SMAX1 domains that are responsible for its nuclear localization, KAR-induced degradation, association with KAI2, and ability to interact with other SMXL proteins. KAI2 undergoes MAX2-independent degradation after KAR treatment, which we propose results from its association with SMAX1 and SMXL2. Finally, we discover an SMXL domain that mediates receptor–target interaction preferences in KAR and SL signaling, laying the foundation for understanding how these highly similar pathways evolved to fulfill different roles.
中文翻译:

SMAX1 的结构功能分析揭示了介导其 Karrikin 诱导的蛋白水解以及与受体 KAI2 相互作用的结构域。
Karrikins (KAR) 是烟雾中发现的丁烯内酯,可以影响许多植物的发芽和幼苗发育。据推测,KAR 信号传导机制与植物激素独脚金内酯 (SL) 非常相似。两条途径都需要 F-box 蛋白 MORE AXILLARY GROWTH2 (MAX2),并且其他核心信号传导成分具有共同的祖先。据推测,KAR 激活受体 KARRIKIN INSENSITIVE2 (KAI2),触发其与 E3 泛素连接酶复合物 SCF MAX2的关联下游目标为 SUPPRESSOR OF MAX2 1 (SMAX1) 和 SMAX1-LIKE2 (SMXL2)。然后,SMAX1 和 SMXL2 的多泛素化和蛋白水解使生长能够响应 KAR。然而,该模型的许多假设尚未得到证实。因此,我们研究了模式植物拟南芥(Arabidopsis thaliana)中SMAX1的翻译后调控。我们发现 SMAX1 被 KAI2-SCF MAX2降解的证据但也受到 MAX2 独立营业额的影响。我们确定了 SMAX1 结构域负责其核定位、KAR 诱导的降解、与 KAI2 的关联以及与其他 SMXL 蛋白相互作用的能力。KAI2 在 KAR 处理后经历了不依赖于 MAX2 的降解,我们认为这是其与 SMAX1 和 SMXL2 相关的结果。最后,我们发现了一个 SMXL 结构域,可以介导 KAR 和 SL 信号传导中受体-靶点相互作用的偏好,为理解这些高度相似的通路如何进化以发挥不同的作用奠定了基础。

























 京公网安备 11010802027423号
京公网安备 11010802027423号