当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fast and Stable N-Terminal Cysteine Modification through Thiazolidino Boronate Mediated Acyl Transfer.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-05-21 , DOI: 10.1002/anie.202000837 Kaicheng Li 1 , Wenjian Wang 1 , Jianmin Gao 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-05-21 , DOI: 10.1002/anie.202000837 Kaicheng Li 1 , Wenjian Wang 1 , Jianmin Gao 1
Affiliation
|
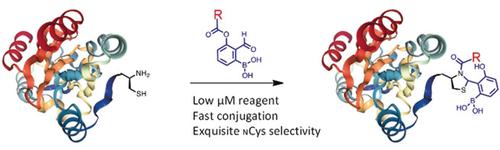
We report a novel conjugation of N‐terminal cysteines (NCys) that proceeds with fast kinetics and exquisite selectivity, thereby enabling facile modification of NCys‐bearing proteins in complex biological milieu. This new NCys conjugation proceeds via a thiazolidine boronate (TzB) intermediate that results from fast (k 2: ≈5000 m −1 s−1) and reversible conjugation of NCys with 2‐formylphenylboronic acid (FPBA). We designed a FPBA derivative that upon TzB formation elicits intramolecular acyl transfer to give N‐acyl thiazolidines. In contrast to the quick hydrolysis of TzB, the N‐acylated thiazolidines exhibit robust stability under physiologic conditions. The utility of the TzB‐mediated NCys conjugation is demonstrated by rapid and non‐disruptive labeling of two enzymes. Furthermore, applying this chemistry to bacteriophage allows facile chemical modification of phage libraries, which greatly expands the chemical space amenable to phage display.
中文翻译:

通过噻唑烷基硼酸酯介导的酰基转移进行快速稳定的 N 端半胱氨酸修饰。
我们报告了一种 N 端半胱氨酸 (NCys) 的新型偶联物,该偶联物具有快速动力学和精细的选择性,从而能够在复杂的生物环境中轻松修饰携带 NCys 的蛋白质。这种新的 NCys 共轭通过噻唑烷硼酸酯 (TzB) 中间体进行,该中间体由快速 ( k 2 : ≈5000 m -1 s -1) 和 NCys 与 2-甲酰基苯基硼酸 (FPBA) 的可逆共轭。我们设计了一种 FPBA 衍生物,它在 TzB 形成时引发分子内酰基转移,得到 N-酰基噻唑烷。与 TzB 的快速水解相反,N-酰化噻唑烷在生理条件下表现出强大的稳定性。TzB 介导的 NCys 偶联的效用通过两种酶的快速和非破坏性标记来证明。此外,将这种化学应用于噬菌体可以轻松地对噬菌体库进行化学修饰,这大大扩展了适合噬菌体展示的化学空间。
更新日期:2020-05-21
中文翻译:

通过噻唑烷基硼酸酯介导的酰基转移进行快速稳定的 N 端半胱氨酸修饰。
我们报告了一种 N 端半胱氨酸 (NCys) 的新型偶联物,该偶联物具有快速动力学和精细的选择性,从而能够在复杂的生物环境中轻松修饰携带 NCys 的蛋白质。这种新的 NCys 共轭通过噻唑烷硼酸酯 (TzB) 中间体进行,该中间体由快速 ( k 2 : ≈5000 m -1 s -1) 和 NCys 与 2-甲酰基苯基硼酸 (FPBA) 的可逆共轭。我们设计了一种 FPBA 衍生物,它在 TzB 形成时引发分子内酰基转移,得到 N-酰基噻唑烷。与 TzB 的快速水解相反,N-酰化噻唑烷在生理条件下表现出强大的稳定性。TzB 介导的 NCys 偶联的效用通过两种酶的快速和非破坏性标记来证明。此外,将这种化学应用于噬菌体可以轻松地对噬菌体库进行化学修饰,这大大扩展了适合噬菌体展示的化学空间。


























 京公网安备 11010802027423号
京公网安备 11010802027423号