当前位置:
X-MOL 学术
›
J. Struct. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exaptation of two ancient immune proteins into a new dimeric pore-forming toxin in snails.
Journal of Structural Biology ( IF 3 ) Pub Date : 2020-05-21 , DOI: 10.1016/j.jsb.2020.107531 M L Giglio 1 , S Ituarte 1 , V Milesi 2 , M S Dreon 1 , T R Brola 1 , J Caramelo 3 , J C H Ip 4 , S Maté 1 , J W Qiu 4 , L H Otero 5 , H Heras 6
Journal of Structural Biology ( IF 3 ) Pub Date : 2020-05-21 , DOI: 10.1016/j.jsb.2020.107531 M L Giglio 1 , S Ituarte 1 , V Milesi 2 , M S Dreon 1 , T R Brola 1 , J Caramelo 3 , J C H Ip 4 , S Maté 1 , J W Qiu 4 , L H Otero 5 , H Heras 6
Affiliation
|
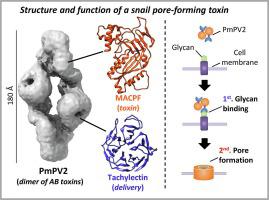
The Membrane Attack Complex-Perforin (MACPF) family is ubiquitously found in all kingdoms. They have diverse cellular roles, however MACPFs with pore-forming toxic function in venoms and poisons are very rare in animals. Here we present the structure of PmPV2, a MACPF toxin from the poisonous apple snail eggs, that can affect the digestive and nervous systems of potential predators. We report the three-dimensional structure of PmPV2, at 17.2 Å resolution determined by negative-stain electron microscopy and its solution structure by small angle X-ray scattering (SAXS). We found that PV2s differ from nearly all MACPFs in two respects: it is a dimer in solution and protomers combine two immune proteins into an AB toxin. The MACPF chain is linked by a single disulfide bond to a tachylectin chain, and two heterodimers are arranged head-to-tail by non-covalent forces in the native protein. MACPF domain is fused with a putative new Ct-accessory domain exclusive to invertebrates. The tachylectin is a six-bladed β-propeller, similar to animal tectonins. We experimentally validated the predicted functions of both subunits and demonstrated for the first time that PV2s are true pore-forming toxins. The tachylectin ¨B¨ delivery subunit would bind to target membranes, and then the MACPF ¨A¨ toxic subunit would disrupt lipid bilayers forming large pores altering the plasma membrane conductance. These results indicate that PV2s toxicity evolved by linking two immune proteins where their combined preexisting functions gave rise to a new toxic entity with a novel role in defense against predation. This structure is an unparalleled example of protein exaptation.
中文翻译:

两种古老的免疫蛋白在蜗牛体内转化为一种新的二聚体成孔毒素。
膜攻击复合物穿孔素 (MACPF) 家族在所有王国中无处不在。它们具有多种细胞作用,但是在毒液和毒物中具有成孔毒性功能的 MACPF 在动物中非常罕见。在这里,我们展示了 PmPV2 的结构,这是一种来自有毒苹果蜗牛卵的 MACPF 毒素,可以影响潜在捕食者的消化系统和神经系统。我们报告了 PmPV2 的三维结构,其分辨率为 17.2 Å,由负染色电子显微镜确定,其溶液结构由小角 X 射线散射 (SAXS) 确定。我们发现 PV2s 在两个方面与几乎所有的 MACPFs 不同:它是溶液中的二聚体,原聚体将两种免疫蛋白结合成 AB 毒素。MACPF 链通过单个二硫键连接到 tachylectin 链,两个异源二聚体通过天然蛋白质中的非共价力头对尾排列。MACPF 域与推定的无脊椎动物独有的新 Ct 附件域融合。速凝素是一种六叶 β 螺旋桨,类似于动物构造蛋白。我们通过实验验证了两个亚基的预测功能,并首次证明 PV2 是真正的成孔毒素。速凝素-B-递送亚基会与靶膜结合,然后MACPF-毒性亚基会破坏脂质双层,形成大孔,改变质膜电导。这些结果表明 PV2s 的毒性是通过连接两种免疫蛋白而进化的,它们的组合预先存在的功能产生了一种新的毒性实体,在防御捕食方面具有新的作用。
更新日期:2020-05-21
中文翻译:

两种古老的免疫蛋白在蜗牛体内转化为一种新的二聚体成孔毒素。
膜攻击复合物穿孔素 (MACPF) 家族在所有王国中无处不在。它们具有多种细胞作用,但是在毒液和毒物中具有成孔毒性功能的 MACPF 在动物中非常罕见。在这里,我们展示了 PmPV2 的结构,这是一种来自有毒苹果蜗牛卵的 MACPF 毒素,可以影响潜在捕食者的消化系统和神经系统。我们报告了 PmPV2 的三维结构,其分辨率为 17.2 Å,由负染色电子显微镜确定,其溶液结构由小角 X 射线散射 (SAXS) 确定。我们发现 PV2s 在两个方面与几乎所有的 MACPFs 不同:它是溶液中的二聚体,原聚体将两种免疫蛋白结合成 AB 毒素。MACPF 链通过单个二硫键连接到 tachylectin 链,两个异源二聚体通过天然蛋白质中的非共价力头对尾排列。MACPF 域与推定的无脊椎动物独有的新 Ct 附件域融合。速凝素是一种六叶 β 螺旋桨,类似于动物构造蛋白。我们通过实验验证了两个亚基的预测功能,并首次证明 PV2 是真正的成孔毒素。速凝素-B-递送亚基会与靶膜结合,然后MACPF-毒性亚基会破坏脂质双层,形成大孔,改变质膜电导。这些结果表明 PV2s 的毒性是通过连接两种免疫蛋白而进化的,它们的组合预先存在的功能产生了一种新的毒性实体,在防御捕食方面具有新的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号