Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2020-05-21 , DOI: 10.1016/j.jinorgbio.2020.111120 Francisco Zárate-Pérez 1 , John C Hackett 1
|
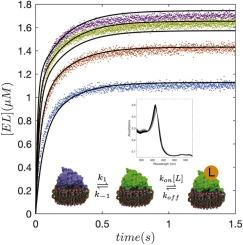
Cytochromes P450 (CYPs) display remarkable plasticity in their ability to bind substrates and catalyze a broad array of chemical reactions. Herein we evaluate binding of androstenedione, testosterone, and 7-hydroxyflavone to CYP19A1, also known as aromatase, in phospholipid nanodiscs by stopped-flow UV–vis spectroscopy. Exponential fitting of the kinetic traces supports the possibility of a multi-step binding mechanism. Subsequent global fitting of the data to the solutions of the coupled differential equations describing the fundamental mechanisms of induced fit and conformational selection, consistently support presence of the latter. To our knowledge, this is the first discrimination of conformational selection from induced fit for a mono-disperse CYP in a native-like membrane environment. In addition, 7-hydroxyflavone binds to CYP19A1 nanodiscs with comparable affinity to the substrates and induces an unusual spectral response likely attributable to hydrogen bonding to, rather than displacement of the heme-coordinated water molecule.
中文翻译:

构象选择存在于与细胞色素 P450 19A1 脂蛋白纳米圆盘结合的配体中。
细胞色素 P450 (CYPs) 在结合底物和催化各种化学反应的能力方面显示出显着的可塑性。在此,我们通过停流紫外-可见光谱评估了磷脂纳米盘中雄烯二酮、睾酮和 7-羟基黄酮与 CYP19A1(也称为芳香酶)的结合。动力学轨迹的指数拟合支持多步结合机制的可能性。随后将数据全局拟合到描述诱导拟合和构象选择基本机制的耦合微分方程的解,一致支持后者的存在。据我们所知,这是构象选择与天然样膜环境中单分散 CYP 的诱导拟合的第一次区分。此外,



























 京公网安备 11010802027423号
京公网安备 11010802027423号