Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Label-free on chip quality assessment of cellular blood products using real-time deformability cytometry.
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-05-20 , DOI: 10.1039/d0lc00258e Konstanze Aurich 1 , Bob Fregin , Raghavendra Palankar , Jan Wesche , Oliver Hartwich , Doreen Biedenweg , Thi-Huong Nguyen , Andreas Greinacher , Oliver Otto
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-05-20 , DOI: 10.1039/d0lc00258e Konstanze Aurich 1 , Bob Fregin , Raghavendra Palankar , Jan Wesche , Oliver Hartwich , Doreen Biedenweg , Thi-Huong Nguyen , Andreas Greinacher , Oliver Otto
Affiliation
|
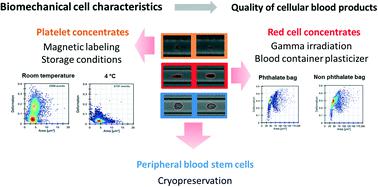
Without cellular blood products such as platelet concentrates (PC), red blood cell concentrates (RCC), and hematopoietic stem cells (HPSC) modern treatments in medicine would not be possible. An unresolved challenge is the assessment of their quality with minimal cell manipulation. Minor changes in production, storage conditions, or blood bag composition may impact cell function, which can have important consequences on product integrity. This is especially relevant for personalized medicine, such as autologous T-cell therapy. Today a robust methodology that globally determines cell status directly before transfusion or transplantation is lacking. We demonstrate that measuring viscoelastic characteristics of peripheral blood cells using real-time deformability cytometry (RT-DC) provides comprehensive information on product quality, which is not accessible using conventional quality control tests. In addition, RT-DC requires few cells, a minimal sample volume and has a rapid turnaround time. We compared RT-DC to standard in vitro quality assays assessing: i) PC after storage at 4 °C and room temperature; ii) magnetic nanoparticle labeled platelets; iii) RCC stored in blood bags with different plasticizers; iv) RCC after gamma irradiation; and v) HPSC after cryopreservation with 5% or 10% dimethyl sulfoxide, respectively. Additionally, we evaluated the engraftment time of patients' platelets and leukocytes after transplantation of HPSC products. Our results demonstrate that label-free mechano-phenotyping can be used as a potential biomarker for quality assessment of cell-based pharmaceutical products.
中文翻译:

使用实时可变形性细胞术对细胞血液产品进行无标签芯片质量评估。
如果没有诸如血小板浓缩液(PC),红细胞浓缩液(RCC)和造血干细胞(HPSC)之类的细胞血液产品,那么医学上的现代治疗将是不可能的。一个尚未解决的挑战是用最少的细胞操作来评估其质量。生产,储存条件或血袋成分的微小变化可能影响细胞功能,这可能对产品的完整性产生重要影响。这对于个性化医学(例如自体T细胞疗法)尤其重要。如今,缺乏一种可以在输血或移植前直接确定细胞状态的强大方法。我们证明,使用实时可变形细胞术(RT-DC)测量外周血细胞的粘弹性特征可提供有关产品质量的全面信息,使用常规质量控制测试无法访问。此外,RT-DC需要很少的样品池,最小的样品量并具有快速的周转时间。我们将RT-DC与标准进行了比较评估体外质量的方法:i)在4°C和室温下保存后的PC;ii)磁性纳米颗粒标记的血小板;iii)将RCC储存在装有不同增塑剂的血袋中;iv)伽马射线辐照后的RCC;v)分别用5%或10%的二甲基亚砜冷冻保存后的HPSC。此外,我们评估了HPSC产品移植后患者血小板和白细胞的移植时间。我们的结果表明,无标签的机械表型可以用作潜在的生物标志物,用于基于细胞的药品质量评估。
更新日期:2020-06-30
中文翻译:

使用实时可变形性细胞术对细胞血液产品进行无标签芯片质量评估。
如果没有诸如血小板浓缩液(PC),红细胞浓缩液(RCC)和造血干细胞(HPSC)之类的细胞血液产品,那么医学上的现代治疗将是不可能的。一个尚未解决的挑战是用最少的细胞操作来评估其质量。生产,储存条件或血袋成分的微小变化可能影响细胞功能,这可能对产品的完整性产生重要影响。这对于个性化医学(例如自体T细胞疗法)尤其重要。如今,缺乏一种可以在输血或移植前直接确定细胞状态的强大方法。我们证明,使用实时可变形细胞术(RT-DC)测量外周血细胞的粘弹性特征可提供有关产品质量的全面信息,使用常规质量控制测试无法访问。此外,RT-DC需要很少的样品池,最小的样品量并具有快速的周转时间。我们将RT-DC与标准进行了比较评估体外质量的方法:i)在4°C和室温下保存后的PC;ii)磁性纳米颗粒标记的血小板;iii)将RCC储存在装有不同增塑剂的血袋中;iv)伽马射线辐照后的RCC;v)分别用5%或10%的二甲基亚砜冷冻保存后的HPSC。此外,我们评估了HPSC产品移植后患者血小板和白细胞的移植时间。我们的结果表明,无标签的机械表型可以用作潜在的生物标志物,用于基于细胞的药品质量评估。


























 京公网安备 11010802027423号
京公网安备 11010802027423号