当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Proton-Coupled Electron Transport in Two Distinct CYBASC Paralogs of Arabidopsis thaliana: A Comparative Characterization of Highly Conserved Tyrosine and Lysine Residues.
Biochemistry ( IF 2.9 ) Pub Date : 2020-05-19 , DOI: 10.1021/acs.biochem.0c00155 Martin Klein 1 , Erhan Deniz 2 , Sabine Heit 1 , Georg Wille 2 , Werner Mäntele 2 , C Roy D Lancaster 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-05-19 , DOI: 10.1021/acs.biochem.0c00155 Martin Klein 1 , Erhan Deniz 2 , Sabine Heit 1 , Georg Wille 2 , Werner Mäntele 2 , C Roy D Lancaster 1
Affiliation
|
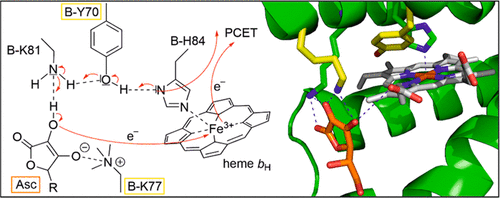
CYBASC proteins are ascorbate (AscH–) reducible, diheme b-containing integral membrane cytochrome b561 proteins (cytb561), which are proposed to be involved in AscH– recycling and facilitation of iron absorption. Two distinct CYBASC paralogs from the plant Arabidopsis thaliana, Atcytb561-A (A-paralog) and Atcytb561-B (B-paralog), have been found to differ in their visible-spectral characteristics and their interaction with AscH– and ferric iron chelates. A previously determined crystal structure of the B-paralog provides the first insights into the structural organization of a CYBASC member and implies hydrogen bonding between the substrate AscH– and the conserved lysine residues at positions 77 (B-K77) and 81 (B-K81). The function of the highly conserved tyrosine at position 70 (B-Y70) is not obvious in the crystal structure, but its localization indicates the possible involvement in proton-coupled electron transfer. Here we show that B-Y70 plays a major role in the modulation of the oxidation–reduction midpoint potential of the high-potential heme, EM(bH), as well as in AscH– oxidation. Our results support the involvement of the functionally conserved B-K77 in the stabilization of the dianion Asc2–. These findings are supported by the crystal structure of the B-paralog, but a comparative biochemical and biophysical characterization of the A- and B-paralogs implied distinct and more complex functions of the corresponding residues A-Y69 and A-K76 in the A-paralog. Our results emphasize the need for a high-resolution crystal structure of the A-paralog to illuminate the differences in functional organization between the two paralogs.
中文翻译:

拟南芥的两个不同的CYBASC旁系同源物中的质子耦合电子传输:高度保守的酪氨酸和赖氨酸残基的比较特征。
CYBASC蛋白是抗坏血酸(ASCH - )还原,diheme b含完整的膜的细胞色素b 561克的蛋白质(细胞色素b 561),其被提出参与ASCH -回收和铁的吸收提供便利。从植物两个不同CYBASC旁系同源物的拟南芥,在细胞色素b 561 -A(A-旁系)和在细胞色素b 561 -B(B-旁系同源),已经发现在它们的可见光谱特性及其与ASCH相互作用而不同–和铁螯合物。的B-旁系同源物的先前确定的晶体结构提供了第一洞察CYBASC构件的结构组织和意味着衬底ASCH之间的氢键-和在位置77(B-K77)和81(B-K81的保守的赖氨酸残基)。在位置70(B-Y70)上高度保守的酪氨酸的功能在晶体结构中并不明显,但其定位表明可能参与了质子耦合电子的转移。在这里,我们表明,B-Y70,起着高电位血红素氧化还原中点电位的调节中起主要作用ê中号(b ^ h),以及在阿希-氧化。我们的结果支持功能上保守的B-K77参与双阴离子Asc 2–的稳定化。这些发现得到了B-旁系同源物的晶体结构的支持,但是A-和B-旁系同源物的比较生化和生物物理表征暗示了A-中相应残基A-Y69和A-K76的独特而又复杂的功能。旁白 我们的结果强调需要A旁系同源物的高分辨率晶体结构,以阐明两个旁系同源物之间功能性组织的差异。
更新日期:2020-06-30
中文翻译:

拟南芥的两个不同的CYBASC旁系同源物中的质子耦合电子传输:高度保守的酪氨酸和赖氨酸残基的比较特征。
CYBASC蛋白是抗坏血酸(ASCH - )还原,diheme b含完整的膜的细胞色素b 561克的蛋白质(细胞色素b 561),其被提出参与ASCH -回收和铁的吸收提供便利。从植物两个不同CYBASC旁系同源物的拟南芥,在细胞色素b 561 -A(A-旁系)和在细胞色素b 561 -B(B-旁系同源),已经发现在它们的可见光谱特性及其与ASCH相互作用而不同–和铁螯合物。的B-旁系同源物的先前确定的晶体结构提供了第一洞察CYBASC构件的结构组织和意味着衬底ASCH之间的氢键-和在位置77(B-K77)和81(B-K81的保守的赖氨酸残基)。在位置70(B-Y70)上高度保守的酪氨酸的功能在晶体结构中并不明显,但其定位表明可能参与了质子耦合电子的转移。在这里,我们表明,B-Y70,起着高电位血红素氧化还原中点电位的调节中起主要作用ê中号(b ^ h),以及在阿希-氧化。我们的结果支持功能上保守的B-K77参与双阴离子Asc 2–的稳定化。这些发现得到了B-旁系同源物的晶体结构的支持,但是A-和B-旁系同源物的比较生化和生物物理表征暗示了A-中相应残基A-Y69和A-K76的独特而又复杂的功能。旁白 我们的结果强调需要A旁系同源物的高分辨率晶体结构,以阐明两个旁系同源物之间功能性组织的差异。


























 京公网安备 11010802027423号
京公网安备 11010802027423号