当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Small Molecule Inhibitors Targeting the Interaction of Ricin Toxin A Subunit with Ribosomes.
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-05-19 , DOI: 10.1021/acsinfecdis.0c00127 Xiao-Ping Li 1 , Rajesh K Harijan 2 , Jennifer N Kahn 1 , Vern L Schramm 2 , Nilgun E Tumer 1
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-05-19 , DOI: 10.1021/acsinfecdis.0c00127 Xiao-Ping Li 1 , Rajesh K Harijan 2 , Jennifer N Kahn 1 , Vern L Schramm 2 , Nilgun E Tumer 1
Affiliation
|
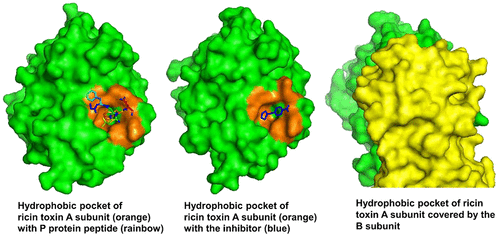
Ricin toxin A subunit (RTA) removes an adenine from the universally conserved sarcin/ricin loop (SRL) on eukaryotic ribosomes, thereby inhibiting protein synthesis. No high affinity and selective small molecule therapeutic antidotes have been reported against ricin toxicity. RTA binds to the ribosomal P stalk to access the SRL. The interaction anchors RTA to the P protein C-termini at a well-defined hydrophobic pocket, which is on the opposite face relative to the active site. The RTA ribosome binding site has not been previously targeted by small molecule inhibitors. We used fragment screening with surface plasmon resonance to identify small molecular weight lead compounds that bind RTA and defined their interactions by crystallography. We identified five fragments, which bound RTA with mid-micromolar affinity. Three chemically distinct binding fragments were cocrystallized with RTA, and crystal structures were solved. Two fragments bound at the P stalk binding site, and the third bound to helix D, a motif distinct from the P stalk binding site. All fragments bound RTA remote from the catalytic site and caused little change in catalytic site geometry. Two fragments uniquely bound at the hydrophobic pocket with affinity sufficient to inhibit the catalytic activity on eukaryotic ribosomes in the low micromolar range. The binding mode of these inhibitors mimicked the interaction of the P stalk peptide, establishing that small molecule inhibitors can inhibit RTA binding to the ribosome with the potential for therapeutic intervention.
中文翻译:

靶向蓖麻毒素A亚基与核糖体相互作用的小分子抑制剂。
蓖麻毒素A亚基(RTA)从真核生物核糖体上普遍保守的sarcin / ricin环(SRL)中去除腺嘌呤,从而抑制蛋白质合成。没有针对蓖麻毒蛋白毒性的高亲和力和选择性小分子解毒剂的报道。RTA绑定到核糖体P茎以访问SRL。相互作用将RTA锚定在相对于活性位点相反的面上的明确疏水口袋处的P蛋白C末端。RTA核糖体结合位点以前尚未被小分子抑制剂靶向。我们使用具有表面等离振子共振的片段筛选技术来鉴定与RTA结合并通过晶体学确定其相互作用的小分子量先导化合物。我们鉴定了五个片段,它们以中等的微摩尔亲和力结合了RTA。将三个化学上不同的结合片段与RTA共结晶,并解析晶体结构。两个片段在P茎结合位点结合,第三个与螺旋D结合,螺旋D与P茎结合位点不同。所有片段都在远离催化部位的RTA上结合,并且催化部位的几何形状几乎没有变化。两个片段在疏水口袋处独特地结合,其亲和力足以在低微摩尔范围内抑制对真核生物核糖体的催化活性。这些抑制剂的结合模式模仿了P茎肽的相互作用,证实了小分子抑制剂可以抑制RTA与核糖体的结合,具有治疗干预的潜力。第三个与螺旋D结合,螺旋D与P茎结合位点不同。所有片段都在远离催化部位的RTA上结合,并且催化部位的几何形状几乎没有变化。两个片段在疏水口袋处独特地结合,其亲和力足以在低微摩尔范围内抑制对真核生物核糖体的催化活性。这些抑制剂的结合模式模仿了P茎肽的相互作用,证实了小分子抑制剂可以抑制RTA与核糖体的结合,具有治疗干预的潜力。第三个与螺旋D结合,螺旋D与P茎结合位点不同。所有片段都在远离催化部位的RTA上结合,并且催化部位的几何形状几乎没有变化。两个片段在疏水口袋处独特地结合,其亲和力足以在低微摩尔范围内抑制对真核生物核糖体的催化活性。这些抑制剂的结合模式模仿了P茎肽的相互作用,建立了小分子抑制剂可以抑制RTA与核糖体的结合,具有治疗干预的潜力。两个片段在疏水口袋处独特地结合,其亲和力足以在低微摩尔范围内抑制对真核生物核糖体的催化活性。这些抑制剂的结合模式模仿了P茎肽的相互作用,建立了小分子抑制剂可以抑制RTA与核糖体的结合,具有治疗干预的潜力。两个片段在疏水口袋处独特地结合,其亲和力足以在低微摩尔范围内抑制对真核生物核糖体的催化活性。这些抑制剂的结合模式模仿了P茎肽的相互作用,建立了小分子抑制剂可以抑制RTA与核糖体的结合,具有治疗干预的潜力。
更新日期:2020-07-10
中文翻译:

靶向蓖麻毒素A亚基与核糖体相互作用的小分子抑制剂。
蓖麻毒素A亚基(RTA)从真核生物核糖体上普遍保守的sarcin / ricin环(SRL)中去除腺嘌呤,从而抑制蛋白质合成。没有针对蓖麻毒蛋白毒性的高亲和力和选择性小分子解毒剂的报道。RTA绑定到核糖体P茎以访问SRL。相互作用将RTA锚定在相对于活性位点相反的面上的明确疏水口袋处的P蛋白C末端。RTA核糖体结合位点以前尚未被小分子抑制剂靶向。我们使用具有表面等离振子共振的片段筛选技术来鉴定与RTA结合并通过晶体学确定其相互作用的小分子量先导化合物。我们鉴定了五个片段,它们以中等的微摩尔亲和力结合了RTA。将三个化学上不同的结合片段与RTA共结晶,并解析晶体结构。两个片段在P茎结合位点结合,第三个与螺旋D结合,螺旋D与P茎结合位点不同。所有片段都在远离催化部位的RTA上结合,并且催化部位的几何形状几乎没有变化。两个片段在疏水口袋处独特地结合,其亲和力足以在低微摩尔范围内抑制对真核生物核糖体的催化活性。这些抑制剂的结合模式模仿了P茎肽的相互作用,证实了小分子抑制剂可以抑制RTA与核糖体的结合,具有治疗干预的潜力。第三个与螺旋D结合,螺旋D与P茎结合位点不同。所有片段都在远离催化部位的RTA上结合,并且催化部位的几何形状几乎没有变化。两个片段在疏水口袋处独特地结合,其亲和力足以在低微摩尔范围内抑制对真核生物核糖体的催化活性。这些抑制剂的结合模式模仿了P茎肽的相互作用,证实了小分子抑制剂可以抑制RTA与核糖体的结合,具有治疗干预的潜力。第三个与螺旋D结合,螺旋D与P茎结合位点不同。所有片段都在远离催化部位的RTA上结合,并且催化部位的几何形状几乎没有变化。两个片段在疏水口袋处独特地结合,其亲和力足以在低微摩尔范围内抑制对真核生物核糖体的催化活性。这些抑制剂的结合模式模仿了P茎肽的相互作用,建立了小分子抑制剂可以抑制RTA与核糖体的结合,具有治疗干预的潜力。两个片段在疏水口袋处独特地结合,其亲和力足以在低微摩尔范围内抑制对真核生物核糖体的催化活性。这些抑制剂的结合模式模仿了P茎肽的相互作用,建立了小分子抑制剂可以抑制RTA与核糖体的结合,具有治疗干预的潜力。两个片段在疏水口袋处独特地结合,其亲和力足以在低微摩尔范围内抑制对真核生物核糖体的催化活性。这些抑制剂的结合模式模仿了P茎肽的相互作用,建立了小分子抑制剂可以抑制RTA与核糖体的结合,具有治疗干预的潜力。

























 京公网安备 11010802027423号
京公网安备 11010802027423号