Free Radical Biology and Medicine ( IF 7.4 ) Pub Date : 2020-05-20 , DOI: 10.1016/j.freeradbiomed.2020.05.004 Homyra Tasnim 1 , Aaron P Landry 1 , Chelsey R Fontenot 1 , Huangen Ding 1
|
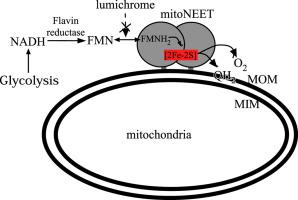
MitoNEET is a mitochondrial outer membrane protein that hosts a redox active [2Fe-2S] cluster in the C-terminal cytosolic domain. Increasing evidence has shown that mitoNEET has an essential role in regulating energy metabolism in human cells. Previously, we reported that the [2Fe-2S] clusters in mitoNEET can be reduced by the reduced flavin mononucleotide (FMNH2) and oxidized by oxygen or ubiquinone-2, suggesting that mitoNEET may act as a novel redox enzyme catalyzing electron transfer from FMNH2 to oxygen or ubiquinone. Here, we explore the FMN binding site in mitoNEET by using FMN analogs and find that lumiflavin, like FMN, at nanomolar concentrations can mediate the redox transition of the mitoNEET [2Fe-2S] clusters in the presence of flavin reductase and NADH (100 μM) under aerobic conditions. The electron paramagnetic resonance (EPR) measurements show that both FMN and lumiflavin can dramatically change the EPR spectrum of the reduced mitoNEET [2Fe-2S] clusters and form a covalently bound complex with mitoNEET under blue light exposure, suggesting that FMN/lumiflavin has specific interactions with the [2Fe-2S] clusters in mitoNEET. In contrast, lumichrome, another FMN analog, fails to mediate the redox transition of the mitoNEET [2Fe-2S] clusters and has no effect on the EPR spectrum of the reduced mitoNEET [2Fe-2S] clusters under blue light exposure. Instead, lumichrome can effectively inhibit the FMNH2-mediated reduction of the mitoNEET [2Fe-2S] clusters, indicating that lumichrome may act as a potential inhibitor to block the electron transfer activity of mitoNEET.
中文翻译:

探索线粒体外膜蛋白mitoNEET中的FMN结合位点。
MitoNEET 是一种线粒体外膜蛋白,在 C 末端胞质结构域中具有氧化还原活性 [2Fe-2S] 簇。越来越多的证据表明,mitoNEET 在调节人体细胞能量代谢方面具有重要作用。此前,我们报道了mitoNEET中的[2Fe-2S]簇可以被还原的黄素单核苷酸(FMNH 2)还原并被氧或泛醌-2氧化,这表明mitoNEET可以作为一种新型氧化还原酶催化FMNH的电子转移2氧气或泛醌。在这里,我们通过使用 FMN 类似物探索 mitoNEET 中的 FMN 结合位点,发现在存在黄素还原酶和 NADH(100 μM ) 在有氧条件下。电子顺磁共振 (EPR) 测量结果表明,FMN 和 lumiflavin 都可以显着改变还原 mitoNEET [2Fe-2S] 簇的 EPR 光谱,并在蓝光照射下与 mitoNEET 形成共价结合的复合物,表明 FMN/lumiflavin 具有特异性与 mitoNEET 中的 [2Fe-2S] 簇的相互作用。相比之下,另一种 FMN 类似物 lumichrome,未能介导 mitoNEET [2Fe-2S] 簇的氧化还原转变,并且对蓝光照射下还原的 mitoNEET [2Fe-2S] 簇的 EPR 光谱没有影响。相反,发光色素可以有效地抑制 FMNH2介导的mitoNEET [2Fe-2S] 簇的还原,表明发光色素可以作为潜在的抑制剂来阻断mitoNEET 的电子转移活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号