Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Efficient Two-Step Protocol for the Isoprenylation of Xanthone at the C2 Position Starting from 1-Fluoroxanthone Derivative
Synlett ( IF 2 ) Pub Date : 2020-05-14 , DOI: 10.1055/s-0039-1690891 Takashi Matsumoto , Yuuki Fujimoto , Chisato Furukawa , Kanae Takahashi , Miho Mochizuki , Hikaru Yanai
Synlett ( IF 2 ) Pub Date : 2020-05-14 , DOI: 10.1055/s-0039-1690891 Takashi Matsumoto , Yuuki Fujimoto , Chisato Furukawa , Kanae Takahashi , Miho Mochizuki , Hikaru Yanai
|
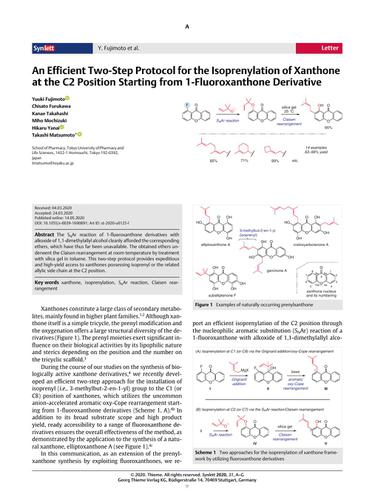
The SNAr reaction of 1-fluoroxanthone derivatives with alkoxide of 1,1-dimethylallyl alcohol cleanly afforded the corresponding ethers, which have thus far been unavailable. The obtained ethers underwent the Claisen rearrangement at room temperature by treatment with silica gel in toluene. This two-step protocol provides expeditious and high-yield access to xanthones possessing isoprenyl or the related allylic side chain at the C2 position.
中文翻译:

从 1-氟氧杂蒽酮衍生物开始对 C2 位置的氧杂蒽酮进行异戊二烯化的高效两步方案
1-氟氧杂蒽酮衍生物与 1,1-二甲基烯丙醇的醇盐的 SNAr 反应干净地提供了相应的醚,迄今为止尚无法获得。通过在甲苯中用硅胶处理,获得的醚在室温下经历克莱森重排。这种两步式协议提供了对在 C2 位置具有异戊二烯基或相关烯丙基侧链的氧杂蒽酮的快速和高产率访问。
更新日期:2020-05-14
中文翻译:

从 1-氟氧杂蒽酮衍生物开始对 C2 位置的氧杂蒽酮进行异戊二烯化的高效两步方案
1-氟氧杂蒽酮衍生物与 1,1-二甲基烯丙醇的醇盐的 SNAr 反应干净地提供了相应的醚,迄今为止尚无法获得。通过在甲苯中用硅胶处理,获得的醚在室温下经历克莱森重排。这种两步式协议提供了对在 C2 位置具有异戊二烯基或相关烯丙基侧链的氧杂蒽酮的快速和高产率访问。


























 京公网安备 11010802027423号
京公网安备 11010802027423号