当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Long-Acting Human Growth Hormone Receptor Antagonists Produced in E. coli and Conjugated with Polyethylene Glycol.
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-05-18 , DOI: 10.1021/acs.bioconjchem.0c00208 Yue Wang 1 , Ries J Langley 2 , Kyle Tamshen 3 , Stephen M Jamieson 2, 4 , Man Lu 1 , Heather D Maynard 3, 5 , Jo K Perry 1, 2
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-05-18 , DOI: 10.1021/acs.bioconjchem.0c00208 Yue Wang 1 , Ries J Langley 2 , Kyle Tamshen 3 , Stephen M Jamieson 2, 4 , Man Lu 1 , Heather D Maynard 3, 5 , Jo K Perry 1, 2
Affiliation
|
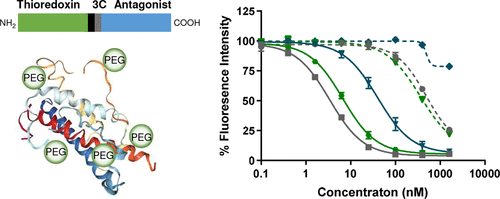
Growth hormone (GH) is a peptide hormone that mediates actions through binding to a cell surface GH receptor (GHR). The GHR antagonist, B2036, combines an amino acid substitution at 120 that confers GHR antagonist activity, with eight additional amino acid substitutions. Conjugation to polyethylene glycol (PEG) increases the serum half-life of these proteins due to reduced renal clearance. Recombinant forms of GH and its antagonists are mainly produced in prokaryotic expression systems, such as E. coli. However, efficient production in E. coli is problematic, as these proteins form aggregates as inclusion bodies resulting in poor solubility. In the present study, we demonstrate that N-terminal fusion to a thioredoxin (Trx) fusion partner improves soluble expression of codon-optimized B2036 in E. coli when expressed at 18 °C. Expression, purification and PEGylation protocols were established for three GHR antagonists: B2036, B20, and G120Rv. Following purification, these antagonists inhibited the proliferation of Ba/F3-GHR cells in a concentration-dependent manner. PEGylation with amine-reactive 5 kDa methoxy PEG succinimidyl propionate yielded a heterogeneous mixture of conjugates containing four to seven PEG moieties. PEGylation significantly reduced in vitro bioactivity of the conjugates. However, substitution of lysine to arginine at amino acid residue 120 in B2036 improved the in vitro activity of the PEGylated protein when compared to unmodified PEGylated B2036. Pharmacokinetic analysis demonstrated that the circulating half-life of PEGylated B20 was 15.2 h in mice. Taken together, we describe an effective strategy to produce biologically active PEGylated human GHR antagonists.
中文翻译:

在大肠杆菌中生产并与聚乙二醇结合的长效人类生长激素受体拮抗剂。
生长激素(GH)是一种肽激素,通过与细胞表面GH受体(GHR)结合而介导作用。GHR拮抗剂B2036将赋予GHR拮抗剂活性的120个氨基酸取代与8个其他氨基酸取代结合起来。由于降低的肾脏清除率,与聚乙二醇(PEG)结合可延长这些蛋白质的血清半衰期。GH及其拮抗剂的重组形式主要在原核表达系统如大肠杆菌中产生。但是,在大肠杆菌中高效生产这是有问题的,因为这些蛋白质形成聚集体作为包涵体,导致溶解性差。在本研究中,我们证明N末端融合到硫氧还蛋白(Trx)融合伙伴可以改善密码子优化的B2036在大肠杆菌中的可溶性表达。在18°C时表示。建立了三种GHR拮抗剂的表达,纯化和PEG化方案:B2036,B20和G120Rv。纯化后,这些拮抗剂以浓度依赖性方式抑制Ba / F3-GHR细胞的增殖。用胺反应性5 kDa甲氧基PEG琥珀酰亚胺基丙酸酯进行PEG化,可得到含有4至7个PEG部分的共轭物的异质混合物。PEG化显着降低了缀合物的体外生物活性。然而,与未修饰的PEG化的B2036相比,B2036中的氨基酸残基120处的赖氨酸被精氨酸取代提高了PEG化的蛋白的体外活性。药代动力学分析表明,PEG化B20在小鼠中的循环半衰期为15.2 h。在一起
更新日期:2020-05-18
中文翻译:

在大肠杆菌中生产并与聚乙二醇结合的长效人类生长激素受体拮抗剂。
生长激素(GH)是一种肽激素,通过与细胞表面GH受体(GHR)结合而介导作用。GHR拮抗剂B2036将赋予GHR拮抗剂活性的120个氨基酸取代与8个其他氨基酸取代结合起来。由于降低的肾脏清除率,与聚乙二醇(PEG)结合可延长这些蛋白质的血清半衰期。GH及其拮抗剂的重组形式主要在原核表达系统如大肠杆菌中产生。但是,在大肠杆菌中高效生产这是有问题的,因为这些蛋白质形成聚集体作为包涵体,导致溶解性差。在本研究中,我们证明N末端融合到硫氧还蛋白(Trx)融合伙伴可以改善密码子优化的B2036在大肠杆菌中的可溶性表达。在18°C时表示。建立了三种GHR拮抗剂的表达,纯化和PEG化方案:B2036,B20和G120Rv。纯化后,这些拮抗剂以浓度依赖性方式抑制Ba / F3-GHR细胞的增殖。用胺反应性5 kDa甲氧基PEG琥珀酰亚胺基丙酸酯进行PEG化,可得到含有4至7个PEG部分的共轭物的异质混合物。PEG化显着降低了缀合物的体外生物活性。然而,与未修饰的PEG化的B2036相比,B2036中的氨基酸残基120处的赖氨酸被精氨酸取代提高了PEG化的蛋白的体外活性。药代动力学分析表明,PEG化B20在小鼠中的循环半衰期为15.2 h。在一起


























 京公网安备 11010802027423号
京公网安备 11010802027423号