当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
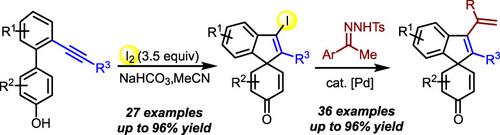
Construction of Alkenyl‐Functionalized Spirocarbocyclic Scaffolds from Alkyne‐Containing Phenol‐Based Biaryls via Sequential Iodine‐Induced Cyclization/Dearomatization and Pd‐Catalyzed Coupling of N‐Tosylhydrazones
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-05-19 , DOI: 10.1002/cjoc.202000170 Anjia Liu 1 , Kaiming Han 1 , Xin‐Xing Wu 1 , Shufeng Chen 1 , Jianbo Wang 2
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-05-19 , DOI: 10.1002/cjoc.202000170 Anjia Liu 1 , Kaiming Han 1 , Xin‐Xing Wu 1 , Shufeng Chen 1 , Jianbo Wang 2
Affiliation
|
An efficient strategy for the formation of alkenyl‐functionalized spirocarbocyclic scaffolds from alkyne‐containing phenol‐based biaryls via sequential iodine‐induced cyclization/dearomatization and Pd‐catalyzed coupling of N‐tosylhydrazones is developed. The approach provides various spirocarbocyclic compounds in moderate to excellent yields with good functional tolerance. The results also demonstrate the feasibility for the direct cross‐couplings of N‐tosylhydrazones with sterically congested tetrasubstituted alkenyl halides.
中文翻译:

通过顺序的碘诱导的N-甲苯磺酰Cycl的环化/脱芳香化和Pd催化的偶联反应,从含炔基的苯基联芳烃中构建烯基官能化的螺碳环骨架。
提出了一种有效的策略,该方法通过依次由碘诱导的环化/脱芳香化作用和钯催化的N-甲苯磺酰hydr偶联反应,由含炔基的酚基联芳基形成烯基官能化的螺碳环骨架。该方法以中等至优异的产率提供了具有良好功能耐受性的各种螺碳环化合物。结果也证明了N-甲苯磺酰hydr与空间上拥挤的四取代链烯基卤化物直接交叉偶联的可行性。
更新日期:2020-05-19
中文翻译:

通过顺序的碘诱导的N-甲苯磺酰Cycl的环化/脱芳香化和Pd催化的偶联反应,从含炔基的苯基联芳烃中构建烯基官能化的螺碳环骨架。
提出了一种有效的策略,该方法通过依次由碘诱导的环化/脱芳香化作用和钯催化的N-甲苯磺酰hydr偶联反应,由含炔基的酚基联芳基形成烯基官能化的螺碳环骨架。该方法以中等至优异的产率提供了具有良好功能耐受性的各种螺碳环化合物。结果也证明了N-甲苯磺酰hydr与空间上拥挤的四取代链烯基卤化物直接交叉偶联的可行性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号