Structure ( IF 5.7 ) Pub Date : 2020-05-19 , DOI: 10.1016/j.str.2020.04.022 Gemma E Seabright 1 , Christopher A Cottrell 2 , Marit J van Gils 3 , Alessio D'addabbo 4 , David J Harvey 5 , Anna-Janina Behrens 6 , Joel D Allen 4 , Yasunori Watanabe 1 , Nicole Scaringi 2 , Thomas M Polveroni 2 , Allison Maker 6 , Snezana Vasiljevic 6 , Natalia de Val 7 , Rogier W Sanders 8 , Andrew B Ward 2 , Max Crispin 1
|
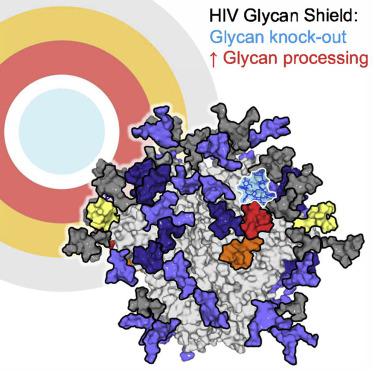
Numerous broadly neutralizing antibodies (bnAbs) have been identified that target the glycans of the HIV-1 envelope spike. Neutralization breadth is notable given that glycan processing can be substantially influenced by the presence or absence of neighboring glycans. Here, using a stabilized recombinant envelope trimer, we investigate the degree to which mutations in the glycan network surrounding an epitope impact the fine glycan processing of antibody targets. Using cryo-electron microscopy and site-specific glycan analysis, we reveal the importance of glycans in the formation of the 2G12 bnAb epitope and show that the epitope is only subtly impacted by variations in the glycan network. In contrast, we show that the PG9 and PG16 glycan-based epitopes at the trimer apex are dependent on the presence of the highly conserved surrounding glycans. Glycan networks underpin the conservation of bnAb epitopes and are an important parameter in immunogen design.
中文翻译:

HIV-1信封聚糖网络在添加和删除聚糖的情况下保持抗体表位。
已鉴定出许多靶向HIV-1包膜刺突的聚糖的广泛中和抗体(bnAb)。考虑到聚糖加工可能受到相邻聚糖的存在或不存在的实质影响,中和广度是值得注意的。在这里,我们使用稳定的重组包膜三聚体,研究围绕表位的聚糖网络中的突变影响抗体靶标的精细聚糖加工的程度。使用冷冻电子显微镜和特定位置的聚糖分析,我们揭示了聚糖在2G12 bnAb表位形成中的重要性,并表明该表位仅受聚糖网络变异的影响。相反,我们显示三聚体顶点处基于PG9和PG16聚糖的表位取决于高度保守的周围聚糖的存在。


























 京公网安备 11010802027423号
京公网安备 11010802027423号