Structure ( IF 5.7 ) Pub Date : 2020-05-19 , DOI: 10.1016/j.str.2020.04.020 Corey D Seacrist 1 , Georg Kuenze 2 , Reece M Hoffmann 3 , Brandon E Moeller 3 , John E Burke 3 , Jens Meiler 4 , Raymond D Blind 5
|
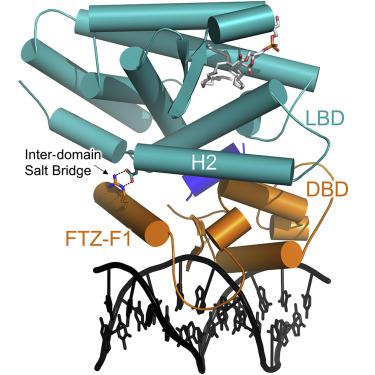
Liver receptor homolog-1 (LRH-1; NR5A2) is a nuclear receptor that regulates a diverse array of biological processes. In contrast to dimeric nuclear receptors, LRH-1 is an obligate monomer and contains a subtype-specific helix at the C terminus of the DNA-binding domain (DBD), termed FTZ-F1. Although detailed structural information is available for individual domains of LRH-1, it is unknown how these domains exist in the intact nuclear receptor. Here, we developed an integrated structural model of human full-length LRH-1 using a combination of HDX-MS, XL-MS, Rosetta computational docking, and SAXS. The model predicts the DBD FTZ-F1 helix directly interacts with ligand binding domain helix 2. We confirmed several other predicted inter-domain interactions via structural and functional analyses. Comparison between the LRH-1/Dax-1 co-crystal structure and the integrated model predicted and confirmed Dax-1 co-repressor to modulate LRH-1 inter-domain dynamics. Together, these data support individual LRH-1 domains interacting to influence receptor structure and function.
中文翻译:

全长LRH-1的集成结构模型揭示了域间相互作用,该相互作用有助于受体的结构和功能。
肝受体同系物1(LRH-1; NR5A2)是调节多种生物过程的核受体。与二聚体核受体相反,LRH-1是专性单体,在DNA结合域(DBD)的C末端包含亚型特异性螺旋,称为FTZ-F1。尽管详细的结构信息可用于LRH-1的各个结构域,但未知这些结构域如何存在于完整的核受体中。在这里,我们使用HDX-MS,XL-MS,Rosetta计算对接和SAXS的组合开发了人类全长LRH-1的集成结构模型。该模型预测DBD FTZ-F1螺旋直接与配体结合域螺旋2相互作用。我们通过结构和功能分析证实了其他几个预测的域间相互作用。LRH-1 / Dax-1共晶体结构与集成模型之间的比较预测并证实了Dax-1共抑制子调节LRH-1域间动力学。这些数据一起支持相互作用的各个LRH-1结构域,以影响受体的结构和功能。

























 京公网安备 11010802027423号
京公网安备 11010802027423号