Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-05-19 , DOI: 10.1016/j.jmb.2020.05.007 Pavel Brázda 1 , Magdaléna Krejčíková 1 , Aiste Kasiliauskaite 1 , Eliška Šmiřáková 1 , Tomáš Klumpler 1 , Robert Vácha 1 , Karel Kubíček 1 , Richard Štefl 1
|
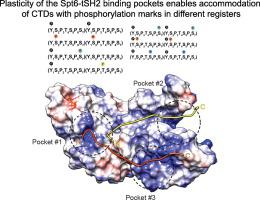
Transcription elongation factor Spt6 associates with RNA polymerase II (RNAP II) via a tandem SH2 (tSH2) domain. The mechanism and significance of the RNAP II–Spt6 interaction is still unclear. Recently, it was proposed that Spt6-tSH2 is recruited via a newly described phosphorylated linker between the Rpb1 core and its C-terminal domain (CTD). Here, we report binding studies with isolated tSH2 of Spt6 (Spt6-tSH2) and Spt6 lacking the first unstructured 297 residues (Spt6ΔN) with a minimal CTD substrate of two repetitive heptads phosphorylated at different sites. The data demonstrate that Spt6 also binds the phosphorylated CTD, a site that was originally proposed as a recognition epitope. We also show that an extended CTD substrate harboring 13 repetitive heptads of the tyrosine-phosphorylated CTD binds Spt6-tSH2 and Spt6ΔN with tighter affinity than the minimal CTD substrate. The enhanced binding is achieved by avidity originating from multiple phosphorylation marks present in the CTD. Interestingly, we found that the steric effects of additional domains in the Spt6ΔN construct partially obscure the binding of the tSH2 domain to the multivalent ligand. We show that Spt6-tSH2 binds various phosphorylation patterns in the CTD and found that the studied combinations of phospho-CTD marks (1,2; 1,5; 2,4; and 2,7) all facilitate the interaction of CTD with Spt6. Our structural studies reveal a plasticity of the tSH2 binding pockets that enables the accommodation of CTDs with phosphorylation marks in different registers.
中文翻译:

酵母Spt6体外读取RNA聚合酶II C末端域的多种磷酸化模式。
转录延伸因子Spt6通过串联SH2(tSH2)域与RNA聚合酶II(RNAP II)缔合。RNAP II–Spt6相互作用的机制和意义仍不清楚。最近,有人提议通过以下方式招募Spt6-tSH2:Rpb1核心与其C末端结构域(CTD)之间的一个新描述的磷酸化连接子。在这里,我们报告与Spt6(Spt6-tSH2)的分离的tSH2和缺乏第一个非结构化的297个残基(Spt6ΔN)的Spt6的结合研究,该残基的最小CTD底物是在不同位点磷酸化的两个重复七聚体。数据表明,Spt6还结合了磷酸化的CTD,该位点最初被提议为识别表位。我们还显示,包含13个酪氨酸磷酸化CTD的7个重复七聚体的扩展CTD底物以最小的CTD底物更紧密的亲和力结合Spt6-tSH2和Spt6ΔN。通过源自CTD中存在的多个磷酸化标记的亲和力来实现增强的结合。有趣的是 我们发现,Spt6ΔN构建体中其他结构域的空间效应部分掩盖了tSH2结构域与多价配体的结合。我们显示,Spt6-tSH2结合了CTD中的各种磷酸化模式,并发现磷酸CTD标记的研究组合(1,2; 1,5; 2,4; 2,7)都促进了CTD与Spt6的相互作用。 。我们的结构研究揭示了tSH2结合口袋的可塑性,使带有磷酸化标记的CTD可以容纳在不同的寄存器中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号