当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Surface Composition Dependence on the Ice Nucleating Ability of Potassium-Rich Feldspar
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-05-18 , DOI: 10.1021/acsearthspacechem.0c00077 Jingwei Yun 1 , Nicole Link 1 , Anand Kumar 1 , Andrey Shchukarev 2 , Jon Davidson 3 , Anita Lam 1 , Christopher Walters 1 , Yu Xi 1 , Jean-Francois Boily 2 , Allan K. Bertram 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-05-18 , DOI: 10.1021/acsearthspacechem.0c00077 Jingwei Yun 1 , Nicole Link 1 , Anand Kumar 1 , Andrey Shchukarev 2 , Jon Davidson 3 , Anita Lam 1 , Christopher Walters 1 , Yu Xi 1 , Jean-Francois Boily 2 , Allan K. Bertram 1
Affiliation
|
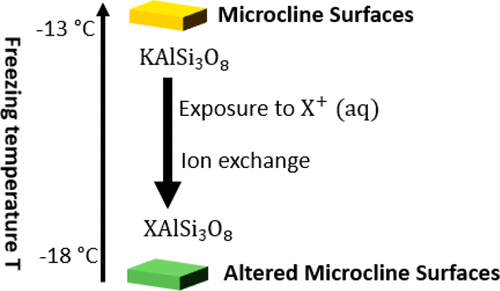
Mineral dust particles are one of the most abundant types of ice nucleating particles in the atmosphere. During atmospheric transport, these particles can be coated with water-soluble solutes, which can modify their ice nucleating ability. Although previous studies have shown that even low concentrations of water-soluble solutes can modify the ice nucleating properties of mineral dust particles, our understanding of this topic is far from complete. We examined the effects of a series of alkali metal nitrates at low concentrations (5 × 10–5 M to 5 × 10–3 M) on the surface composition and immersion freezing of potassium-rich feldspar (K-rich feldspar). Immersion freezing was investigated with the droplet freezing technique, and the surface composition was investigated with cryogenic X-ray photoelectron spectroscopy. K+ increased the median freezing temperature of the droplets, while the other alkali metal cations either had no effect or decreased the median freezing temperature. The changes in the median freezing temperature of the droplets due to the presence of nitrates followed the order K+ ≥ Li+ ≥ Na+ ≥ Rb+ ≥ Cs+ and, except for Cs+, were correlated to the K/Al ratio at the surface of K-rich feldspar. The K/Al ratio is possibly an indicator of the abundance of certain types of K-bearing microcline surfaces that drive the immersion freezing of K-rich feldspar, while Cs+ likely influences the immersion freezing of K-rich feldspar by an additional mechanism, possibly blocking ice nucleation sites by adsorption. Our work also shows that the cation charge density (charge density over the surface area of a single cation) is not a good predictor of the effects of cations on the immersion freezing of K-rich feldspar in our experiments.
中文翻译:

表面成分对富钾长石冰成核能力的依赖
矿物尘埃颗粒是大气中最丰富的冰核颗粒类型之一。在大气运输过程中,这些颗粒可以涂有水溶性溶质,从而可以改变其冰核能力。尽管以前的研究表明,即使是低浓度的水溶性溶质也可以改变矿物粉尘颗粒的冰成核特性,但我们对这一主题的理解还远远不够。我们研究了一系列低浓度(5×10 –5 M至5×10 –3的碱金属硝酸盐的影响M)在表面上组成并浸没冷冻富钾长石(K-rich长石)。用液滴冷冻技术研究浸没冷冻,并用低温X射线光电子能谱研究表面组成。K +增加了液滴的中值冻结温度,而其他碱金属阳离子则没有影响或降低了中值冻结温度。由于存在硝酸盐,液滴的中值冻结温度的变化遵循K + ≥Li + ≥Na + ≥Rb + ≥Cs +的顺序,除了Cs +,与富钾长石表面的钾/铝比相关。钾/铝比可能表明某些类型的含钾微晶表面的丰度,这些表面驱动富钾长石的浸没冻结,而Cs +可能通过其他机制影响富钾长石的浸没冻结,可能会通过吸附阻止冰成核位置。我们的工作还表明,在我们的实验中,阳离子电荷密度(单个阳离子表面积上的电荷密度)不能很好地预测阳离子对富钾长石浸没冻结的影响。
更新日期:2020-06-18
中文翻译:

表面成分对富钾长石冰成核能力的依赖
矿物尘埃颗粒是大气中最丰富的冰核颗粒类型之一。在大气运输过程中,这些颗粒可以涂有水溶性溶质,从而可以改变其冰核能力。尽管以前的研究表明,即使是低浓度的水溶性溶质也可以改变矿物粉尘颗粒的冰成核特性,但我们对这一主题的理解还远远不够。我们研究了一系列低浓度(5×10 –5 M至5×10 –3的碱金属硝酸盐的影响M)在表面上组成并浸没冷冻富钾长石(K-rich长石)。用液滴冷冻技术研究浸没冷冻,并用低温X射线光电子能谱研究表面组成。K +增加了液滴的中值冻结温度,而其他碱金属阳离子则没有影响或降低了中值冻结温度。由于存在硝酸盐,液滴的中值冻结温度的变化遵循K + ≥Li + ≥Na + ≥Rb + ≥Cs +的顺序,除了Cs +,与富钾长石表面的钾/铝比相关。钾/铝比可能表明某些类型的含钾微晶表面的丰度,这些表面驱动富钾长石的浸没冻结,而Cs +可能通过其他机制影响富钾长石的浸没冻结,可能会通过吸附阻止冰成核位置。我们的工作还表明,在我们的实验中,阳离子电荷密度(单个阳离子表面积上的电荷密度)不能很好地预测阳离子对富钾长石浸没冻结的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号