当前位置:
X-MOL 学术
›
Eur. J. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Trigger-dependent differences determine therapeutic outcome in murine primary hemophagocytic lymphohistiocytosis.
European Journal of Immunology ( IF 5.4 ) Pub Date : 2020-05-17 , DOI: 10.1002/eji.201948123 Ruth Gather 1, 2 , Peter Aichele 3 , Nadja Goos 1 , Jan Rohr 1, 4 , Hanspeter Pircher 3 , Tamara Kögl 3 , Robert Zeiser 5 , Hartmut Hengel 6 , Annette Schmitt-Gräff 7 , Casey Weaver 8 , Stephan Ehl 1
European Journal of Immunology ( IF 5.4 ) Pub Date : 2020-05-17 , DOI: 10.1002/eji.201948123 Ruth Gather 1, 2 , Peter Aichele 3 , Nadja Goos 1 , Jan Rohr 1, 4 , Hanspeter Pircher 3 , Tamara Kögl 3 , Robert Zeiser 5 , Hartmut Hengel 6 , Annette Schmitt-Gräff 7 , Casey Weaver 8 , Stephan Ehl 1
Affiliation
|
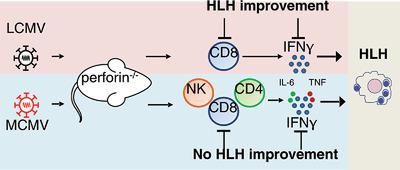
Familial hemophagocytic lymphohistiocytosis (FHL) is a hyperinflammatory syndrome affecting patients with genetic cytotoxicity defects. Perforin‐deficient (PKO) mice recapitulate the full clinical picture of FHL after infection with lymphocytic choriomeningitis virus (LCMV). Hyperactivated CD8+ T cells and IFN‐γ have been identified as the key drivers of FHL and represent targets for therapeutic interventions. However, the response of patients is variable. This could be due to trigger‐dependent differences in pathogenesis, which is difficult to address in FHL patients, since the trigger frequently escapes detection. We established an alternative FHL model using intravenous infection of PKO mice with murine CMV (MCMV)Smith. PKO mice developed acute FHL after both infections and fulfilled HLH diagnostic criteria accompanied by excessive IFN‐γ production by disease‐inducing T cells, that enrich in the BM. However, direct comparison of the two infection models disclosed trigger‐dependence of FHL progression and revealed a higher contribution of CD4 T cells and NK cells to IFN‐γ production after MCMV infection. Importantly, therapeutic intervention by IFN‐γ neutralization or CD8+ T‐cell depletion had less benefit in MCMV‐triggered FHL compared to LCMV‐triggered FHL, likely due to MCMV‐induced cytopathology. Thus, the context of the specific triggering viral infection can impact the success of targeted immunotherapeutic HLH control.
中文翻译:

触发依赖的差异决定了鼠原发性吞噬淋巴细胞组织细胞增生的治疗结果。
家族性噬血细胞淋巴组织细胞增生症(FHL)是一种高炎症综合症,会影响具有遗传细胞毒性缺陷的患者。Perforin缺陷(PKO)小鼠概述了淋巴细胞性脉络膜脑膜炎病毒(LCMV)感染后FHL的全部临床情况。高活化的CD8 + T细胞和IFN-γ已被确定为FHL的主要驱动力,并代表了治疗干预的目标。但是,患者的反应是可变的。这可能是由于发病机制中依赖于触发因素的差异所致,而这在FHL患者中很难解决,因为触发因素经常无法被发现。我们使用鼠CMV(MCMV)Smith的PKO小鼠静脉感染建立了替代的FHL模型。PKO小鼠在两次感染后均发展为急性FHL,并且符合HLH诊断标准,并伴随疾病诱导性T细胞过量产生IFN-γ,从而丰富了BM。然而,两种感染模型的直接比较揭示了FHL进展的触发依赖性,并揭示了MCMV感染后CD4 T细胞和NK细胞对IFN-γ产生的贡献更大。重要的是,与MCMV触发的FHL相比,通过IFNγ中和或CD8 + T细胞耗竭进行的治疗干预与MCMV触发的FHL相比,对MCMV触发的FHL的获益较少,这可能是由于MCMV诱导的细胞病理学所致。因此,特异性触发病毒感染的情况会影响靶向免疫治疗HLH控制的成功。
更新日期:2020-05-17
中文翻译:

触发依赖的差异决定了鼠原发性吞噬淋巴细胞组织细胞增生的治疗结果。
家族性噬血细胞淋巴组织细胞增生症(FHL)是一种高炎症综合症,会影响具有遗传细胞毒性缺陷的患者。Perforin缺陷(PKO)小鼠概述了淋巴细胞性脉络膜脑膜炎病毒(LCMV)感染后FHL的全部临床情况。高活化的CD8 + T细胞和IFN-γ已被确定为FHL的主要驱动力,并代表了治疗干预的目标。但是,患者的反应是可变的。这可能是由于发病机制中依赖于触发因素的差异所致,而这在FHL患者中很难解决,因为触发因素经常无法被发现。我们使用鼠CMV(MCMV)Smith的PKO小鼠静脉感染建立了替代的FHL模型。PKO小鼠在两次感染后均发展为急性FHL,并且符合HLH诊断标准,并伴随疾病诱导性T细胞过量产生IFN-γ,从而丰富了BM。然而,两种感染模型的直接比较揭示了FHL进展的触发依赖性,并揭示了MCMV感染后CD4 T细胞和NK细胞对IFN-γ产生的贡献更大。重要的是,与MCMV触发的FHL相比,通过IFNγ中和或CD8 + T细胞耗竭进行的治疗干预与MCMV触发的FHL相比,对MCMV触发的FHL的获益较少,这可能是由于MCMV诱导的细胞病理学所致。因此,特异性触发病毒感染的情况会影响靶向免疫治疗HLH控制的成功。


























 京公网安备 11010802027423号
京公网安备 11010802027423号