当前位置:
X-MOL 学术
›
Microbiologyopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Penicillin-binding protein PBP2a provides variable levels of protection toward different β-lactams in Staphylococcus aureus RN4220.
MicrobiologyOpen ( IF 3.4 ) Pub Date : 2020-05-17 , DOI: 10.1002/mbo3.1057 Marte Ekeland Fergestad 1, 2 , Gro Anita Stamsås 1 , Danae Morales Angeles 1 , Zhian Salehian 1 , Yngvild Wasteson 2 , Morten Kjos 1
MicrobiologyOpen ( IF 3.4 ) Pub Date : 2020-05-17 , DOI: 10.1002/mbo3.1057 Marte Ekeland Fergestad 1, 2 , Gro Anita Stamsås 1 , Danae Morales Angeles 1 , Zhian Salehian 1 , Yngvild Wasteson 2 , Morten Kjos 1
Affiliation
|
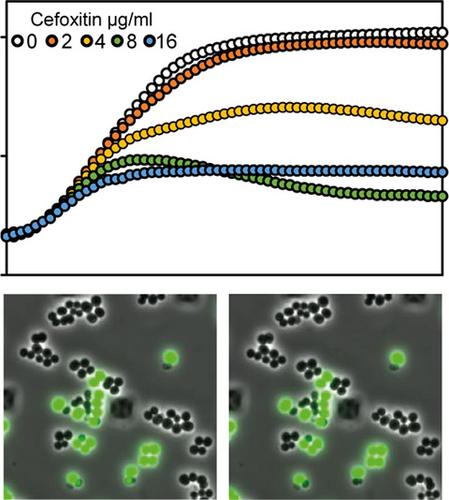
Methicillin‐resistant Staphylococcus aureus (MRSA) is resistant to most β‐lactams due to the expression of an extra penicillin‐binding protein, PBP2a, with low β‐lactam affinity. It has long been known that heterologous expression of the PBP2a‐encoding mecA gene in methicillin‐sensitive S. aureus (MSSA) provides protection towards β‐lactams, however, some reports suggest that the degree of protection can vary between different β‐lactams. To test this more systematically, we introduced an IPTG‐inducible mecA into the MSSA laboratory strain RN4220. We confirm, by growth assays as well as single‐cell microfluidics time‐lapse microscopy experiments, that PBP2a expression protects against β‐lactams in S. aureus RN4220. By testing a panel of ten different β‐lactams, we conclude that there is also a great variation in the level of protection conferred by PBP2a. Expression of PBP2a resulted in an only fourfold increase in minimum inhibitory concentration (MIC) for imipenem, while a 32‐fold increase in MIC was observed for cefaclor and cephalexin. Interestingly, in our experimental setup, PBP2a confers the highest protection against cefaclor and cephalexin—two β‐lactams that are known to have a high specific affinity toward the transpeptidase PBP3 of S. aureus. Notably, using a single‐cell microfluidics setup we demonstrate a considerable phenotypic variation between cells upon β‐lactam exposure and show that mecA‐expressing S. aureus can survive β‐lactam concentrations much higher than the minimal inhibitory concentrations. We discuss possible explanations and implications of these results including important aspects regarding treatment of infection.
中文翻译:

青霉素结合蛋白PBP2a对金黄色葡萄球菌RN4220中的不同β-内酰胺提供不同程度的保护。
耐甲氧西林金黄色葡萄球菌(MRSA)对大多数β-内酰胺具有抗性,这是由于表达了一种额外的青霉素结合蛋白PBP2a,其β-内酰胺亲和力低。早就知道在甲氧西林敏感的金黄色葡萄球菌(MSSA)中编码PBP2a的mecA基因的异源表达可提供针对β-内酰胺的保护,但是,一些报道表明,保护程度可能因不同的β-内酰胺而异。为了更系统地进行测试,我们将IPTG诱导型mecA引入了MSSA实验室菌株RN4220。通过生长分析以及单细胞微流控延时显微镜实验,我们证实了PBP2a表达可以保护金黄色葡萄球菌中的β-内酰胺。RN4220。通过测试一组十种不同的β-内酰胺,我们得出结论,PBP2a赋予的保护水平也存在很大差异。PBP2a的表达导致亚胺培南的最低抑菌浓度(MIC)仅增加四倍,而头孢克洛和头孢氨苄的MIC则增加32倍。有趣的是,在我们的实验装置中,PBP2a对头孢克洛和头孢氨苄具有最高保护作用-两种β-内酰胺已知对金黄色葡萄球菌的转肽酶PBP3具有高特异性亲和力。值得注意的是,使用单细胞微流控装置,我们证明了暴露于β-内酰胺的细胞之间存在明显的表型变异,并表明表达mecA的金黄色葡萄球菌β-内酰胺浓度可以比最小抑菌浓度高得多。我们讨论了这些结果的可能解释和含义,包括有关感染治疗的重要方面。
更新日期:2020-05-17
中文翻译:

青霉素结合蛋白PBP2a对金黄色葡萄球菌RN4220中的不同β-内酰胺提供不同程度的保护。
耐甲氧西林金黄色葡萄球菌(MRSA)对大多数β-内酰胺具有抗性,这是由于表达了一种额外的青霉素结合蛋白PBP2a,其β-内酰胺亲和力低。早就知道在甲氧西林敏感的金黄色葡萄球菌(MSSA)中编码PBP2a的mecA基因的异源表达可提供针对β-内酰胺的保护,但是,一些报道表明,保护程度可能因不同的β-内酰胺而异。为了更系统地进行测试,我们将IPTG诱导型mecA引入了MSSA实验室菌株RN4220。通过生长分析以及单细胞微流控延时显微镜实验,我们证实了PBP2a表达可以保护金黄色葡萄球菌中的β-内酰胺。RN4220。通过测试一组十种不同的β-内酰胺,我们得出结论,PBP2a赋予的保护水平也存在很大差异。PBP2a的表达导致亚胺培南的最低抑菌浓度(MIC)仅增加四倍,而头孢克洛和头孢氨苄的MIC则增加32倍。有趣的是,在我们的实验装置中,PBP2a对头孢克洛和头孢氨苄具有最高保护作用-两种β-内酰胺已知对金黄色葡萄球菌的转肽酶PBP3具有高特异性亲和力。值得注意的是,使用单细胞微流控装置,我们证明了暴露于β-内酰胺的细胞之间存在明显的表型变异,并表明表达mecA的金黄色葡萄球菌β-内酰胺浓度可以比最小抑菌浓度高得多。我们讨论了这些结果的可能解释和含义,包括有关感染治疗的重要方面。



























 京公网安备 11010802027423号
京公网安备 11010802027423号