Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2020-05-16 , DOI: 10.1016/j.jfluchem.2020.109558 Mohamed EL Guendouzi , Jamal Faridi
|
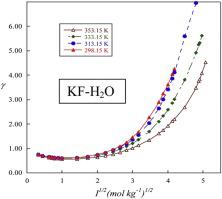
The experimental and calculated solubilities of potassium fluoride in aqueous solutions were investigated at various temperatures. The experimental solubility of KF in aqueous solutions was determined up to mmax = 17.50 to 25.80 mol kg−1 respectively from 298.15 to 353.15 K. The calculated solubility requires the thermodynamic properties of the binary system based on the water activity measurements. Accordingly, the water activity of KF aqueous solutions were measured from diluted to the saturated solutions, using the hygrometric method at different temperatures. The related thermodynamic properties of KF(aq) were evaluated by the development of ion interaction model. The new binary parameters (β(0)(K,F), β(1)(K,F), and Cφ(KF)) were determined at various temperatures and used to evaluate the solute activity and the osmotic coefficients ranging from 298.15 to 353.15 K. The solubility product of KF(s) (given in ) and the standard molar Gibbs energies of dissolution were also given at different temperatures.
中文翻译:

不同温度下氟化钾在水溶液中的热力学性质和溶解度
在不同温度下研究了氟化钾在水溶液中的实验和计算溶解度。确定了KF在水溶液中的实验溶解度,分别从298.15至353.15 K分别高达m max = 17.50至25.80 mol kg -1。计算出的溶解度需要基于水活度测量值的二元体系的热力学性质。因此,使用吸湿法在不同温度下将KF水溶液的水活性从稀释至饱和溶液进行了测定。通过建立离子相互作用模型评价了KF(aq)的相关热力学性质。新的二进制参数(β (0)(K,F),β(1)(K,F) ,和Ç φ (KF) )中在不同温度确定,并用于评估活性溶质和渗透系数从298.15到353.15 K的KF的溶度积(S) (在给定的)和标准的摩尔吉布斯溶解能 也可以在不同温度下服用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号