当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Potency Increase of Spiroketal Analogs of Membrane Inserting Indolyl Mannich Base Antimycobacterials Is Due to Acquisition of MmpL3 Inhibition.
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-05-15 , DOI: 10.1021/acsinfecdis.0c00121 Ming Li 1 , Zheng Yen Phua 2 , Yu Xi 2 , Zhujun Xu 2 , Samuel A Nyantakyi 3 , Wei Li 4 , Mary Jackson 4 , Ming Wah Wong 2 , Yulin Lam 2 , Shu Sin Chng 2, 5 , Mei Lin Go 3 , Thomas Dick 6, 7, 8
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-05-15 , DOI: 10.1021/acsinfecdis.0c00121 Ming Li 1 , Zheng Yen Phua 2 , Yu Xi 2 , Zhujun Xu 2 , Samuel A Nyantakyi 3 , Wei Li 4 , Mary Jackson 4 , Ming Wah Wong 2 , Yulin Lam 2 , Shu Sin Chng 2, 5 , Mei Lin Go 3 , Thomas Dick 6, 7, 8
Affiliation
|
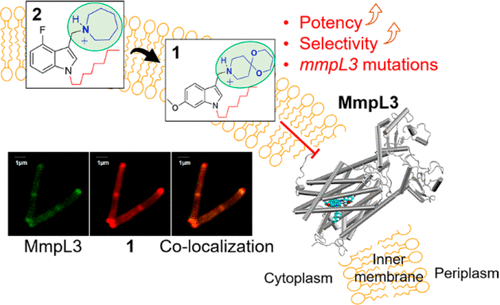
Chemistry campaigns identified amphiphilic indolyl Mannich bases as novel membrane-permeabilizing antimycobacterials. Spiroketal analogs of this series showed increased potency, and the lead compound 1 displayed efficacy in a mouse model of tuberculosis. Yet the mechanism by which the spiroketal moiety accomplished the potency “jump” remained unknown. Consistent with its membrane-permeabilizing mechanism, no resistant mutants could be isolated against indolyl Mannich base 2 lacking the spiroketal moiety. In contrast, mutations resistant against spiroketal analog 1 were obtained in mycobacterial membrane protein large 3 (MmpL3), a proton motive force (PMF)-dependent mycolate transporter. Thus, we hypothesized that the potency jump observed for 1 may be due to MmpL3 inhibition acquired by the addition of the spiroketal moiety. Here we showed that 1 inhibited MmpL3 flippase activity without loss of the PMF, colocalized with MmpL3tb–GFP in intact organisms, and yielded a consistent docking pose within the “common inhibitor binding pocket” of MmpL3. The presence of the spiroketal motif in 1 ostensibly augmented its interaction with MmpL3, an outcome not observed in the nonspiroketal analog 2, which displayed no cross-resistance to mmpL3 mutants, dissipated the PMF, and docked poorly in the MmpL3 binding pocket. Surprisingly, 2 inhibited MmpL3 flippase activity, which may be an epiphenomenon arising from its wider membrane disruptive effects. Hence, we conclude that the potency increase associated with the spiroketal analog 1 is linked to the acquisition of a second mechanism, MmpL3 inhibition. In contrast, the nonspiroketal analog 2 acts pleiotropically, affecting several cell membrane-embedded targets, including MmpL3, through its membrane permeabilizing and depolarizing effects.
中文翻译:

膜插入吲哚基曼尼希碱抗分枝杆菌的螺旋类似物的效能增加是由于获得了MmpL3抑制作用。
化学运动确定了两亲性吲哚基曼尼希碱为新型的膜通透性抗分枝杆菌。该系列的Spirooketal类似物显示出更高的效价,并且铅化合物1在结核病小鼠模型中显示出功效。然而,螺旋体部分完成效力“跳跃”的机制仍是未知的。与它的膜通透性机制一致,无法针对缺乏螺环部分的吲哚基曼尼希碱基2分离出抗性突变体。相比之下,在较大的分枝杆菌膜蛋白3(MmpL3),质子动力(PMF)依赖的霉菌酸酯转运蛋白中获得了对spiroketal类似物1的抗性突变。因此,我们假设观察到1的效力跳跃可能是由于通过添加螺环部分而获得的MmpL3抑制作用。在这里,我们显示了1抑制MmpL3脂肪酶的活性而没有PMF的损失,在完整的生物体中与MmpL3tb-GFP共定位,并且在MmpL3的“常见抑制剂结合口袋”中产生了一致的停靠姿势。螺环基序1的存在从表面上增强了它与MmpL3的相互作用,这在非螺环基类似物2中未观察到,该结果对mmpL3突变体没有交叉抗性,使PMF消散,并且不能很好地对接在MmpL3结合口袋中。令人惊讶的是2抑制MmpL3翻转酶活性,这可能是由于其更广泛的膜破坏作用引起的现象。因此,我们得出结论,与螺环酮类似物1相关的效力增加与第二种机制MmpL3抑制的获得有关。相比之下,非螺环类似物2具有多效性,通过其膜透化和去极化作用影响包括MmpL3在内的多个细胞膜嵌入靶标。
更新日期:2020-07-10
中文翻译:

膜插入吲哚基曼尼希碱抗分枝杆菌的螺旋类似物的效能增加是由于获得了MmpL3抑制作用。
化学运动确定了两亲性吲哚基曼尼希碱为新型的膜通透性抗分枝杆菌。该系列的Spirooketal类似物显示出更高的效价,并且铅化合物1在结核病小鼠模型中显示出功效。然而,螺旋体部分完成效力“跳跃”的机制仍是未知的。与它的膜通透性机制一致,无法针对缺乏螺环部分的吲哚基曼尼希碱基2分离出抗性突变体。相比之下,在较大的分枝杆菌膜蛋白3(MmpL3),质子动力(PMF)依赖的霉菌酸酯转运蛋白中获得了对spiroketal类似物1的抗性突变。因此,我们假设观察到1的效力跳跃可能是由于通过添加螺环部分而获得的MmpL3抑制作用。在这里,我们显示了1抑制MmpL3脂肪酶的活性而没有PMF的损失,在完整的生物体中与MmpL3tb-GFP共定位,并且在MmpL3的“常见抑制剂结合口袋”中产生了一致的停靠姿势。螺环基序1的存在从表面上增强了它与MmpL3的相互作用,这在非螺环基类似物2中未观察到,该结果对mmpL3突变体没有交叉抗性,使PMF消散,并且不能很好地对接在MmpL3结合口袋中。令人惊讶的是2抑制MmpL3翻转酶活性,这可能是由于其更广泛的膜破坏作用引起的现象。因此,我们得出结论,与螺环酮类似物1相关的效力增加与第二种机制MmpL3抑制的获得有关。相比之下,非螺环类似物2具有多效性,通过其膜透化和去极化作用影响包括MmpL3在内的多个细胞膜嵌入靶标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号