当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
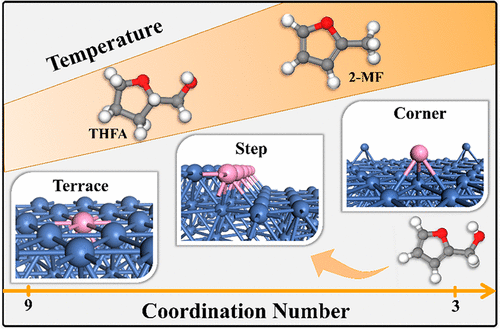
Catalytic Conversion Furfuryl Alcohol to Tetrahydrofurfuryl Alcohol and 2-Methylfuran at Terrace, Step, and Corner Sites on Ni
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-05-15 , DOI: 10.1021/acscatal.0c01441 Lifang Chen 1 , Jingyun Ye 2 , Yusen Yang 1 , Pan Yin 1 , Haisong Feng 1 , Chunyuan Chen 1 , Xin Zhang 1 , Min Wei 1 , Donald G. Truhlar 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-05-15 , DOI: 10.1021/acscatal.0c01441 Lifang Chen 1 , Jingyun Ye 2 , Yusen Yang 1 , Pan Yin 1 , Haisong Feng 1 , Chunyuan Chen 1 , Xin Zhang 1 , Min Wei 1 , Donald G. Truhlar 2
Affiliation
|
The surface structures at catalytic sites are critical factors for determining catalytic selectivity. Here, we use periodic density functional theory and microkinetic modeling to systematically investigate the effect of surface structures on the conversion of furfuryl alcohol (FA). We consider nine surface terminations of Ni with various coordination numbers representing terrace, step, and corner sites. We study three reaction paths for FA conversion on various surfaces and find that the surface structure impacts the adsorption configuration and causes significant differences in selectivity. Barrier height analysis shows that terrace sites favor hydrogenation to tetrahydrofurfuryl alcohol (THFA), whereas corner sites favor C–OH bond scission to produce 2-methylfuran (2-MF); step sites show similar barriers for the two reactions. We explain this by identifying three characteristics of the reactant adsorption structures that have a significant effect on selectivity, namely, that a shorter distance between the adsorbed hydrogen atom and the C3 carbon of FA favors hydrogenation to produce THFA, and more negative charge transfer to Oalcohol and a longer C–Oalcohol bond length favor C–Oalcohol bond scission to produce 2-MF. Since the reactions have similar barriers at a step site, microkinetic calculations are employed to calculate the product selectivity on a step site under experimental conditions. At lower temperatures and higher generalized coordination number (CN), THFA is the most favorable product, while the selectivity to 2-MF is higher at lower CN and at higher temperature. This work provides guidance for the rational design catalysts to control the product distribution of FA conversion.
中文翻译:

在Ni的平台,台阶和拐角处将糠醇催化转化为四氢糠醇和2-甲基呋喃
催化部位的表面结构是确定催化选择性的关键因素。在这里,我们使用周期性密度泛函理论和微动力学模型来系统研究表面结构对糠醇(FA)转化的影响。我们考虑了Ni的9个表面终止点,它们具有代表露台,台阶和拐角位置的各种配位数。我们研究了在各种表面上进行FA转化的三种反应路径,发现表面结构会影响吸附构型并导致选择性显着不同。势垒高度分析表明,梯级位有利于氢化成四氢糠醇(THFA),而拐角位有利于C-OH键断裂,从而生成2-甲基呋喃(2-MF)。台阶部位对两个反应显示出相似的障碍。醇和更长的C–O醇键长度有利于C–O醇键断裂产生2-MF。由于反应在台阶部位具有相似的壁垒,因此采用微动力学计算来计算实验条件下在台阶部位的产物选择性。在较低的温度和较高的广义配位数(CN)下,THFA是最有利的产物,而在较低的CN和较高的温度下,对2-MF的选择性较高。这项工作为合理设计催化剂以控制FA转化产物的分布提供了指导。
更新日期:2020-07-02
中文翻译:

在Ni的平台,台阶和拐角处将糠醇催化转化为四氢糠醇和2-甲基呋喃
催化部位的表面结构是确定催化选择性的关键因素。在这里,我们使用周期性密度泛函理论和微动力学模型来系统研究表面结构对糠醇(FA)转化的影响。我们考虑了Ni的9个表面终止点,它们具有代表露台,台阶和拐角位置的各种配位数。我们研究了在各种表面上进行FA转化的三种反应路径,发现表面结构会影响吸附构型并导致选择性显着不同。势垒高度分析表明,梯级位有利于氢化成四氢糠醇(THFA),而拐角位有利于C-OH键断裂,从而生成2-甲基呋喃(2-MF)。台阶部位对两个反应显示出相似的障碍。醇和更长的C–O醇键长度有利于C–O醇键断裂产生2-MF。由于反应在台阶部位具有相似的壁垒,因此采用微动力学计算来计算实验条件下在台阶部位的产物选择性。在较低的温度和较高的广义配位数(CN)下,THFA是最有利的产物,而在较低的CN和较高的温度下,对2-MF的选择性较高。这项工作为合理设计催化剂以控制FA转化产物的分布提供了指导。


























 京公网安备 11010802027423号
京公网安备 11010802027423号