当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pharmacokinetics and subjective effects of 1P-LSD in humans after oral and intravenous administration.
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2020-06-02 , DOI: 10.1002/dta.2821 Christina Grumann 1 , Kerstin Henkel 1 , Simon D Brandt 2 , Alexander Stratford 3 , Torsten Passie 4, 5 , Volker Auwärter 1
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2020-06-02 , DOI: 10.1002/dta.2821 Christina Grumann 1 , Kerstin Henkel 1 , Simon D Brandt 2 , Alexander Stratford 3 , Torsten Passie 4, 5 , Volker Auwärter 1
Affiliation
|
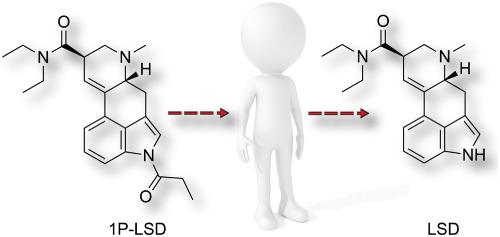
1‐Propanoyl‐lysergic acid diethylamide (1P‐LSD) appeared as a non‐controlled alternative to LSD a few years ago. Although evidence is beginning to emerge from in vitro and animal studies that 1P‐LSD might serve as a prodrug for LSD, an equivalent evaluation in humans is unavailable. Controlled oral and intravenous self‐administrations of 100 μg 1P‐LSD hemitartrate are reported in two human volunteers followed by analyses of urine and serum samples using a fully validated LC–MS/MS method. Psychometric evaluations included assessment of selected subjective drug effects and administration of the Five‐Dimensions of Altered States of Consciousness rating scale (5D‐ASC). In serum and urine, oral administrations of 1P‐LSD only led to the detection of LSD reflecting biphasic elimination with a terminal elimination half‐life of approx. t1/2 = 6.4 h. 1P‐LSD could be detected for only up to 4.16 h in serum and 2.7 h in urine following intravenous administration, whereas LSD was detected in all serum samples (last sampling after approx. 24 h) and up to 80 h in urine. LSD showed first order elimination kinetics with an approx. t1/2 = 5.7 h, whereas 1P‐LSD showed a rapid decrease in concentration within the first hour followed by a slower decrease, most probably due to hydrolysis. The bioavailability of LSD after oral ingestion of 1P‐LSD was close to 100%. The psychosensory effects of 1P‐LSD and their time course were comparable to those seen after uptake of LSD in other studies which further supports the prodrug hypothesis. The 5D‐ASC scores were higher after oral compared with intravenous administration of 1P‐LSD.
中文翻译:

口服和静脉内给药后1P-LSD在人体中的药代动力学和主观效应。
几年前,1-丙酰基麦角酰二乙酰胺(1P-LSD)成为LSD的非受控替代品。尽管从体外和动物研究中开始出现证据表明1P-LSD可能是LSD的前药,但尚无与人体等效的评估方法。在两名人类志愿者中,据报道口服和静脉内自我给药100μg1P-LSD半酒石酸盐的剂量受控,然后使用经过充分验证的LC-MS / MS方法分析尿液和血清样品。心理计量学评估包括对所选主观药物效果的评估以及“知觉改变状态的五个维度”评定量表(5D-ASC)的管理。在血清和尿液中,口服1P-LSD仅能导致LSD的检测,反映出双相消除,终末消除半衰期约为5分钟。Ť1/2 = 6.4小时。静脉内给药后,在血清中最多只能检测到1P‐LSD,在尿液中最多可以检测到4.16 h,而在尿液中最多可以检测到2.7 h,而在所有血清样品中(最后一次采样大约在24 h后)和尿液中最多可以检测到LSD长达80 h。LSD表现出一级消除动力学,约为 t 1/2 = 5.7小时,而1P‐LSD在第一个小时内浓度迅速降低,随后缓慢降低,这很可能是由于水解所致。口服摄入1P-LSD后LSD的生物利用度接近100%。1P‐LSD的心理感觉效果及其时程与其他研究中摄取LSD后所见的效果相当,这进一步支持了前药假说。口服1P-LSD较口服5D-ASC得分高。
更新日期:2020-06-02
中文翻译:

口服和静脉内给药后1P-LSD在人体中的药代动力学和主观效应。
几年前,1-丙酰基麦角酰二乙酰胺(1P-LSD)成为LSD的非受控替代品。尽管从体外和动物研究中开始出现证据表明1P-LSD可能是LSD的前药,但尚无与人体等效的评估方法。在两名人类志愿者中,据报道口服和静脉内自我给药100μg1P-LSD半酒石酸盐的剂量受控,然后使用经过充分验证的LC-MS / MS方法分析尿液和血清样品。心理计量学评估包括对所选主观药物效果的评估以及“知觉改变状态的五个维度”评定量表(5D-ASC)的管理。在血清和尿液中,口服1P-LSD仅能导致LSD的检测,反映出双相消除,终末消除半衰期约为5分钟。Ť1/2 = 6.4小时。静脉内给药后,在血清中最多只能检测到1P‐LSD,在尿液中最多可以检测到4.16 h,而在尿液中最多可以检测到2.7 h,而在所有血清样品中(最后一次采样大约在24 h后)和尿液中最多可以检测到LSD长达80 h。LSD表现出一级消除动力学,约为 t 1/2 = 5.7小时,而1P‐LSD在第一个小时内浓度迅速降低,随后缓慢降低,这很可能是由于水解所致。口服摄入1P-LSD后LSD的生物利用度接近100%。1P‐LSD的心理感觉效果及其时程与其他研究中摄取LSD后所见的效果相当,这进一步支持了前药假说。口服1P-LSD较口服5D-ASC得分高。


























 京公网安备 11010802027423号
京公网安备 11010802027423号