当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Salts and Cocrystal of Etodolac: Advantage of Solubility, Dissolution, and Permeability
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-05-15 , DOI: 10.1021/acs.cgd.0c00313 Sunil K. Rai 1 , Suryanarayana Allu 2 , Ashwini K. Nangia 1, 2
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-05-15 , DOI: 10.1021/acs.cgd.0c00313 Sunil K. Rai 1 , Suryanarayana Allu 2 , Ashwini K. Nangia 1, 2
Affiliation
|
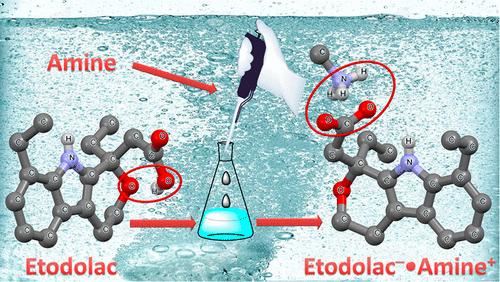
Etodolac (ETD) is a nonsteroidal anti-inflammatory drug (NSAID) approved by the United States Food and Drug Administration (US-FDA) in 1991 for the treatment of rheumatoid arthritis. Because of its poor aqueous solubility and high permeability, ETD falls under Biopharmaceutics Classification System (BCS) Class II drug. The present study was aimed to screen stable salts and cocrystals of ETD using Generally Recognized as Safe (GRAS) and a few non-GRAS coformers. Crystallization of five salts (i.e., isopropylamine = isoPA, n-hexylamine = nHA, cyclohexylamine = cycloHA, 2-phenylethylamine = phEA, piperazine = PPZ) and one cocrystal (isonicotinamide = INT) was successful. These products were characterized by single crystal X-ray and powder diffraction. Differential scanning calorimetry (DSC) showed a single endotherm for the salts, which confirmed their thermal stability and phase homogeneity, except for ETD–·phEA+ where a solid–solid transition at 152 °C was observed with an enthalpy of transition ΔH ≈ 16 J/g. Among the five salts, ETD–·isoPA+ showed the highest solubility of 267.50 mg/mL and ∼20 times faster intrinsic dissolution rate than ETD in pH 7.0 phosphate buffer medium. The salts are stable under solubility and dissolution conditions as confirmed by fitting the powder X-ray diffraction profile of each sample after the experiment with the calculated lines from the X-ray structure. Permeability and flux analysis of ETD salts showed that ETD–·isoPA+ exhibits a high flux rate across the semipermeable membrane due to a higher molecular mobility and greater concentration gradient.
中文翻译:

依托度酸的盐和共结晶:溶解性,溶解性和渗透性的优势
依托度拉克(ETD)是一种非甾体抗炎药(NSAID),于1991年获得美国食品和药物管理局(US-FDA)的批准,用于治疗类风湿性关节炎。由于其不良的水溶性和高渗透性,ETD属于生物制药分类系统(BCS)II类药物。本研究旨在使用“公认安全”(GRAS)和一些非GRAS共形成物来筛选ETD的稳定盐和共结晶。五个盐(结晶即,异丙胺= ISOPA,Ñ己胺=磷灰石,环己胺= cycloHA,2-苯乙胺= PHEA,哌嗪= PPZ)和一个共结晶(异烟酰胺= INT)成功。这些产物通过单晶X射线和粉末衍射表征。差示扫描量热法(DSC)显示出对盐的单个吸热,这证实了它们的热稳定性和相均匀性,除了ETD - ·PHEA +其中具有过渡Δ的焓观察在152℃的固-固转变ħ ≈ 16焦/克。在这五种盐中,ETD – ·isoPA +的最高溶解度为267.50 mg / mL,比ETD快20倍左右。在pH 7.0磷酸盐缓冲液中。这些盐在溶解度和溶解度条件下是稳定的,这是通过将实验后的每个样品的粉末X射线衍射图与X射线结构的计算线拟合而确定的。ETD盐的渗透性和通量分析表明,由于较高的分子迁移率和较大的浓度梯度,ETD – ·isoPA +在整个半透膜上显示出较高的通量率。
更新日期:2020-07-01
中文翻译:

依托度酸的盐和共结晶:溶解性,溶解性和渗透性的优势
依托度拉克(ETD)是一种非甾体抗炎药(NSAID),于1991年获得美国食品和药物管理局(US-FDA)的批准,用于治疗类风湿性关节炎。由于其不良的水溶性和高渗透性,ETD属于生物制药分类系统(BCS)II类药物。本研究旨在使用“公认安全”(GRAS)和一些非GRAS共形成物来筛选ETD的稳定盐和共结晶。五个盐(结晶即,异丙胺= ISOPA,Ñ己胺=磷灰石,环己胺= cycloHA,2-苯乙胺= PHEA,哌嗪= PPZ)和一个共结晶(异烟酰胺= INT)成功。这些产物通过单晶X射线和粉末衍射表征。差示扫描量热法(DSC)显示出对盐的单个吸热,这证实了它们的热稳定性和相均匀性,除了ETD - ·PHEA +其中具有过渡Δ的焓观察在152℃的固-固转变ħ ≈ 16焦/克。在这五种盐中,ETD – ·isoPA +的最高溶解度为267.50 mg / mL,比ETD快20倍左右。在pH 7.0磷酸盐缓冲液中。这些盐在溶解度和溶解度条件下是稳定的,这是通过将实验后的每个样品的粉末X射线衍射图与X射线结构的计算线拟合而确定的。ETD盐的渗透性和通量分析表明,由于较高的分子迁移率和较大的浓度梯度,ETD – ·isoPA +在整个半透膜上显示出较高的通量率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号