当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pharmacological Profile and Molecular Modeling of Cyclic Opioid Analogues Incorporating Various Phenylalanine Derivatives.
ChemMedChem ( IF 3.4 ) Pub Date : 2020-05-15 , DOI: 10.1002/cmdc.202000248 Anna Adamska-Bartłomiejczyk 1 , Piotr F J Lipiński 2 , Justyna Piekielna-Ciesielska 1 , Alicja Kluczyk 3 , Anna Janecka 1
ChemMedChem ( IF 3.4 ) Pub Date : 2020-05-15 , DOI: 10.1002/cmdc.202000248 Anna Adamska-Bartłomiejczyk 1 , Piotr F J Lipiński 2 , Justyna Piekielna-Ciesielska 1 , Alicja Kluczyk 3 , Anna Janecka 1
Affiliation
|
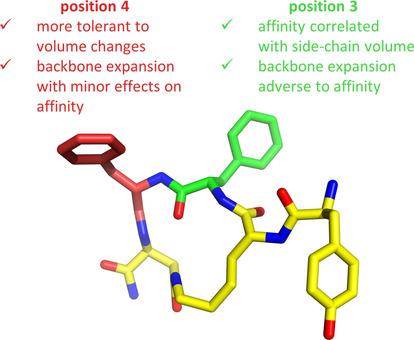
Peptide‐based agonists of the μ opioid receptor (μOR) are promising therapeutic candidates for pain relief with reduced side effects compared to morphine. A deep understanding of μOR–ligand interactions is necessary for future design of peptide‐based opioid analgesics. To explore the requirements of the μOR binding pocket, eight new analogues of our cyclic peptide Tyr‐c[d ‐Lys−Phe−Phe−Asp]NH2 displaying high μOR affinity were synthesized, in which Phe in either the third or fourth position was replaced by various derivatives of this amino acid (β3‐Phe, homoPhe, β3‐homoPhe and PhGly). The aim of this research was to examine the structural effects of such modifications on the bioactivity, and both experimental and theoretical methods were used. The binding of the cyclic analogues to all three OR types (μ, δ, κ) was assessed by radioligand competitive binding assay, and their functional activity was determined in a calcium mobilization assay. In order to provide structural hypotheses explaining the obtained experimental affinities, the complexes of the cyclic peptides with μOR were subjected to molecular modeling.
中文翻译:

含有各种苯丙氨酸衍生物的环状阿片类似物的药理学特征和分子模型。
与吗啡相比,μ阿片受体(μOR)的基于肽的激动剂有望成为缓解疼痛的有希望的治疗药物。对μOR-配体相互作用的深入了解对于未来基于肽的阿片类镇痛药的设计是必要的。为了探索μOR结合口袋的要求,我们合成了八种新的环状肽Tyr-c [ d -Lys-Phe-Phe-Asp] NH 2表现出高μOR亲和力的类似物,其中Phe在第三或第四位置通过这种氨基酸的各种衍生物代替(β 3 -Phe,homoPhe,β 3-homoPhe和PhGly)。这项研究的目的是检查这种修饰对生物活性的结构影响,并使用了实验和理论方法。通过放射性配体竞争结合测定法评估环状类似物与所有三种OR类型(μ,δ,κ)的结合,并通过钙动员测定法测定其功能活性。为了提供解释所获得的实验亲和力的结构假设,对具有μOR的环状肽的复合物进行分子建模。
更新日期:2020-07-20
中文翻译:

含有各种苯丙氨酸衍生物的环状阿片类似物的药理学特征和分子模型。
与吗啡相比,μ阿片受体(μOR)的基于肽的激动剂有望成为缓解疼痛的有希望的治疗药物。对μOR-配体相互作用的深入了解对于未来基于肽的阿片类镇痛药的设计是必要的。为了探索μOR结合口袋的要求,我们合成了八种新的环状肽Tyr-c [ d -Lys-Phe-Phe-Asp] NH 2表现出高μOR亲和力的类似物,其中Phe在第三或第四位置通过这种氨基酸的各种衍生物代替(β 3 -Phe,homoPhe,β 3-homoPhe和PhGly)。这项研究的目的是检查这种修饰对生物活性的结构影响,并使用了实验和理论方法。通过放射性配体竞争结合测定法评估环状类似物与所有三种OR类型(μ,δ,κ)的结合,并通过钙动员测定法测定其功能活性。为了提供解释所获得的实验亲和力的结构假设,对具有μOR的环状肽的复合物进行分子建模。


























 京公网安备 11010802027423号
京公网安备 11010802027423号