当前位置:
X-MOL 学术
›
DNA Repair
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular dissection of Helicobacter pylori Topoisomerase I reveals an additional active site in the carboxyl terminus of the enzyme.
DNA Repair ( IF 3.8 ) Pub Date : 2020-05-15 , DOI: 10.1016/j.dnarep.2020.102853 Sumedha M Kondekar 1 , Gaurav V Gunjal 1 , Juan Pablo Radicella 2 , Desirazu N Rao 1
DNA Repair ( IF 3.8 ) Pub Date : 2020-05-15 , DOI: 10.1016/j.dnarep.2020.102853 Sumedha M Kondekar 1 , Gaurav V Gunjal 1 , Juan Pablo Radicella 2 , Desirazu N Rao 1
Affiliation
|
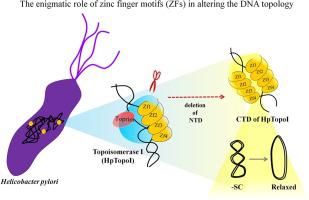
DNA topoisomerases play a crucial role in maintaining DNA superhelicity, thereby regulating various cellular processes. Unlike most other species, the human pathogen Helicobacter pylori has only two topoisomerases, Topoisomerase I and DNA gyrase, the physiological roles of which remain to be explored. Interestingly, there is enormous variability among the C-terminal domains (CTDs) of Topoisomerase I across bacteria. H. pylori Topoisomerase I (HpTopoI) CTD harbors four zinc finger motifs (ZFs). We show here that sequential deletion of the third and/or fourth ZFs had only a marginal effect on the HpTopoI activity, while deletion of the second, third and fourth ZFs severely reduced DNA relaxation activity. Deletion of all ZFs drastically hampered DNA binding and thus abolished DNA relaxation. Surprisingly, mutagenesis of the annotated active site tyrosine residue (Y297 F) did not abrogate the enzyme activity and HpTopoI CTD alone (spanning the four ZFs) showed DNA relaxation activity. Additionally, a covalent linkage between the DNA and HpTopoI CTD was identified. The capacity of HpTopoI CTD to complement Escherichia coli topA mutant strains further supported the in vitro observations. Collectively these results imply that not all ZFs are dispensable for HpTopoI activity and unveil the presence of additional non-canonical catalytic site(s) within the enzyme.
中文翻译:

幽门螺杆菌拓扑异构酶I的分子解剖揭示了该酶羧基末端的另一个活性位点。
DNA拓扑异构酶在维持DNA超螺旋性,从而调节各种细胞过程中起着至关重要的作用。与大多数其他物种不同,人类病原体幽门螺杆菌只有两种拓扑异构酶,拓扑异构酶I和DNA促旋酶,其生理作用尚待探索。有趣的是,拓扑异构酶I的C末端结构域(CTD)之间跨细菌存在巨大差异。幽门螺杆菌拓扑异构酶I(HpTopoI)CTD包含四个锌指基序(ZF)。我们在这里显示,第三和/或第四ZF的顺序删除仅对HpTopoI活性有边际影响,而第二,第三和第四ZF的删除则严重降低了DNA松弛活性。删除所有ZF会严重阻碍DNA结合,从而消除DNA松弛。出奇,对带注释的活性位点酪氨酸残基(Y297 F)的诱变并未消除酶的活性,仅HpTopoI CTD(跨越四个ZF)显示出DNA弛豫活性。另外,鉴定了DNA与HpTopoI CTD之间的共价键。HpTopoI CTD补充大肠杆菌topA突变株的能力进一步支持了体外观察。总的来说,这些结果暗示并非所有ZF对于HpTopoI活性都是可有可无的,并且揭示了酶内另外的非规范催化位点的存在。HpTopoI CTD补充大肠杆菌topA突变株的能力进一步支持了体外观察。总的来说,这些结果暗示并非所有ZF对于HpTopoI活性都是可有可无的,并且揭示了酶内另外的非规范催化位点的存在。HpTopoI CTD补充大肠杆菌topA突变株的能力进一步支持了体外观察。总的来说,这些结果暗示并非所有ZF对于HpTopoI活性都是可有可无的,并且揭示了酶内另外的非规范催化位点的存在。
更新日期:2020-05-15
中文翻译:

幽门螺杆菌拓扑异构酶I的分子解剖揭示了该酶羧基末端的另一个活性位点。
DNA拓扑异构酶在维持DNA超螺旋性,从而调节各种细胞过程中起着至关重要的作用。与大多数其他物种不同,人类病原体幽门螺杆菌只有两种拓扑异构酶,拓扑异构酶I和DNA促旋酶,其生理作用尚待探索。有趣的是,拓扑异构酶I的C末端结构域(CTD)之间跨细菌存在巨大差异。幽门螺杆菌拓扑异构酶I(HpTopoI)CTD包含四个锌指基序(ZF)。我们在这里显示,第三和/或第四ZF的顺序删除仅对HpTopoI活性有边际影响,而第二,第三和第四ZF的删除则严重降低了DNA松弛活性。删除所有ZF会严重阻碍DNA结合,从而消除DNA松弛。出奇,对带注释的活性位点酪氨酸残基(Y297 F)的诱变并未消除酶的活性,仅HpTopoI CTD(跨越四个ZF)显示出DNA弛豫活性。另外,鉴定了DNA与HpTopoI CTD之间的共价键。HpTopoI CTD补充大肠杆菌topA突变株的能力进一步支持了体外观察。总的来说,这些结果暗示并非所有ZF对于HpTopoI活性都是可有可无的,并且揭示了酶内另外的非规范催化位点的存在。HpTopoI CTD补充大肠杆菌topA突变株的能力进一步支持了体外观察。总的来说,这些结果暗示并非所有ZF对于HpTopoI活性都是可有可无的,并且揭示了酶内另外的非规范催化位点的存在。HpTopoI CTD补充大肠杆菌topA突变株的能力进一步支持了体外观察。总的来说,这些结果暗示并非所有ZF对于HpTopoI活性都是可有可无的,并且揭示了酶内另外的非规范催化位点的存在。


























 京公网安备 11010802027423号
京公网安备 11010802027423号