Computational and Structural Biotechnology Journal ( IF 6 ) Pub Date : 2020-05-14 , DOI: 10.1016/j.csbj.2020.05.011 Aleš Buček 1, 2 , Mario Vazdar 3 , Michal Tupec 1 , Aleš Svatoš 1, 4 , Iva Pichová 1
|
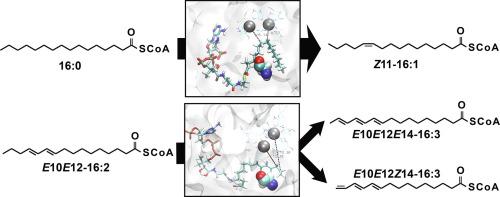
Membrane fatty acyl desaturases (mFAD) are ubiquitous enzymes in eukaryotes. They introduce double bonds into fatty acids (FAs), producing structurally diverse unsaturated FAs which serve as membrane lipid components or precursors of signaling molecules. The mechanisms controlling enzymatic specificity and selectivity of desaturation are, however, poorly understood. We found that the physicochemical properties, particularly side chain volume, of a single amino acid (aa) residue in insect mFADs (Lepidoptera: Bombyx mori and Manduca sexta) control the desaturation products. Molecular dynamics simulations of systems comprising wild-type or mutant mFADs with fatty acyl-CoA substrates revealed that the single aa substitution likely directs the outcome of the desaturation reaction by modulating the distance between substrate fatty acyl carbon atoms and active center metal ions. These findings, as well as our methodology combining mFAD mutational screening with molecular dynamics simulations, will facilitate prediction of desaturation products and facilitate engineering of mFADs for biotechnological applications.
中文翻译:

去饱和酶的特异性受底物结合通道中单个氨基酸残基的物理化学特性控制。
膜脂肪酰基去饱和酶(mFAD)是真核生物中普遍存在的酶。它们将双键引入脂肪酸(FAs)中,从而产生结构多样的不饱和FAs,它们充当膜脂质成分或信号分子的前体。然而,对控制酶特异性和去饱和度选择性的机制了解甚少。我们发现昆虫mFAD中的单个氨基酸(aa)残基的物理化学特性,特别是侧链体积(鳞翅目:家蚕和曼杜卡六性ta))控制去饱和产物。包含具有脂肪酰基辅酶A底物的野生型或突变mFADs的系统的分子动力学模拟显示,单个aa取代可能通过调节底物脂肪酰基碳原子与活性中心金属离子之间的距离来指导去饱和反应的结果。这些发现以及我们将mFAD突变筛选与分子动力学模拟相结合的方法,将有助于去饱和产物的预测,并简化用于生物技术应用的mFAD的工程设计。



























 京公网安备 11010802027423号
京公网安备 11010802027423号