当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modulated Scanning Fluorimetry Can Quickly Assess Thermal Protein Unfolding Reversibility in Microvolume Samples.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-05-13 , DOI: 10.1021/acs.molpharmaceut.0c00330 Hristo L Svilenov 1 , Tim Menzen 2 , Klaus Richter 2 , Gerhard Winter 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-05-13 , DOI: 10.1021/acs.molpharmaceut.0c00330 Hristo L Svilenov 1 , Tim Menzen 2 , Klaus Richter 2 , Gerhard Winter 1
Affiliation
|
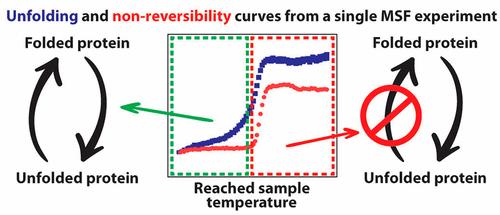
Determining the temperature at which the thermal unfolding of a protein starts becoming irreversible is relevant for many areas of protein research. Until now, published methods cannot determine, within a reasonable time frame and with moderate sample consumption, the exposure temperature that starts causing irreversible protein unfolding. We present modulated scanning fluorimetry (MSF) and share a software (MSF Analyzer), which can be used to derive nonreversibility curves of thermal protein unfolding from a series of incremental temperature cycles performed on only 10 μL samples, consuming as low as a few micrograms of protein. Further processing of the data can yield the onset temperature that starts causing nonreversible protein unfolding. The MSF method is based on the hardware of the already existing nanoDSF technology and can be applied to dozens of samples simultaneously. Here, we use MSF to study how solution pH affects the reversibility of thermal protein unfolding of several model proteins to show that the nonreversibility onset temperature (Tnr) is a unique biophysical parameter, providing orthogonal information from thermal protein denaturation data and insights into the validity of thermal unfolding analysis in the context of equilibrium thermodynamics. We also show that MSF can be used to study enzyme stability after exposure to high temperatures. Besides, we demonstrate that protein thermal unfolding and nonreversibility can be affected in different ways upon modifications like PEG-ylation or labeling with fluorescent dyes. Finally, we show that MSF can be used to study the effect of various protein interactions on thermal protein unfolding reversibility. With the diverse examples in this work, we reveal how MSF can provide orthogonal information from thermal denaturation experiments that can bring benefits to various areas of protein research. The MSF Analyzer software is available at https://github.com/CoriolisPharmaResearch/MSFAnalyser.
中文翻译:

调制扫描荧光法可以快速评估微量样品中蛋白质的热解折叠可逆性。
确定蛋白质热解开开始不可逆的温度与蛋白质研究的许多领域有关。迄今为止,已公开的方法无法在合理的时间范围内并以适度的样品消耗来确定开始引起不可逆的蛋白质展开的暴露温度。我们介绍了调制扫描荧光法(MSF),并共享了一个软件(MSF Analyzer),该软件可用于从仅对10μL样品进行的一系列递增温度循环中得出的热蛋白质展开的不可逆曲线,其消耗量低至几微克。蛋白质。数据的进一步处理可以产生起始温度,该起始温度开始引起不可逆的蛋白质展开。MSF方法基于现有的nanoDSF技术的硬件,可以同时应用于数十个样品。在这里,我们使用MSF研究溶液的pH值如何影响几种模型蛋白的热蛋白展开的可逆性,从而表明不可逆性起始温度(Ť NR)是一个独特的生物物理参数,可提供来自热蛋白质变性数据的正交信息,以及在平衡热力学背景下深入了解热展开分析的有效性的信息。我们还表明,MSF可用于研究暴露于高温后的酶稳定性。此外,我们证明了蛋白质的热解折叠和不可逆性可以通过诸如PEG-基化或荧光染料标记的修饰以不同的方式受到影响。最后,我们表明MSF可用于研究各种蛋白质相互作用对热蛋白质展开可逆性的影响。通过这项工作中的各种示例,我们揭示了MSF如何从热变性实验中提供正交信息,从而可以为蛋白质研究的各个领域带来益处。
更新日期:2020-07-06
中文翻译:

调制扫描荧光法可以快速评估微量样品中蛋白质的热解折叠可逆性。
确定蛋白质热解开开始不可逆的温度与蛋白质研究的许多领域有关。迄今为止,已公开的方法无法在合理的时间范围内并以适度的样品消耗来确定开始引起不可逆的蛋白质展开的暴露温度。我们介绍了调制扫描荧光法(MSF),并共享了一个软件(MSF Analyzer),该软件可用于从仅对10μL样品进行的一系列递增温度循环中得出的热蛋白质展开的不可逆曲线,其消耗量低至几微克。蛋白质。数据的进一步处理可以产生起始温度,该起始温度开始引起不可逆的蛋白质展开。MSF方法基于现有的nanoDSF技术的硬件,可以同时应用于数十个样品。在这里,我们使用MSF研究溶液的pH值如何影响几种模型蛋白的热蛋白展开的可逆性,从而表明不可逆性起始温度(Ť NR)是一个独特的生物物理参数,可提供来自热蛋白质变性数据的正交信息,以及在平衡热力学背景下深入了解热展开分析的有效性的信息。我们还表明,MSF可用于研究暴露于高温后的酶稳定性。此外,我们证明了蛋白质的热解折叠和不可逆性可以通过诸如PEG-基化或荧光染料标记的修饰以不同的方式受到影响。最后,我们表明MSF可用于研究各种蛋白质相互作用对热蛋白质展开可逆性的影响。通过这项工作中的各种示例,我们揭示了MSF如何从热变性实验中提供正交信息,从而可以为蛋白质研究的各个领域带来益处。



























 京公网安备 11010802027423号
京公网安备 11010802027423号