当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aromatic Stacking Facilitated Self-Assembly of Ultrashort Ionic Complementary Peptide Sequence: β-Sheet Nanofibers with Remarkable Gelation and Interfacial Properties.
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-05-13 , DOI: 10.1021/acs.biomac.0c00366 Jacek K Wychowaniec 1, 2, 3 , Ronak Patel 4 , James Leach 4 , Rachel Mathomes 4 , Vikesh Chhabria 4 , Yogita Patil-Sen 4 , Araida Hidalgo-Bastida 5, 6, 7 , Robert T Forbes 4 , Joseph M Hayes 4 , Mohamed A Elsawy 1, 2, 4, 8
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-05-13 , DOI: 10.1021/acs.biomac.0c00366 Jacek K Wychowaniec 1, 2, 3 , Ronak Patel 4 , James Leach 4 , Rachel Mathomes 4 , Vikesh Chhabria 4 , Yogita Patil-Sen 4 , Araida Hidalgo-Bastida 5, 6, 7 , Robert T Forbes 4 , Joseph M Hayes 4 , Mohamed A Elsawy 1, 2, 4, 8
Affiliation
|
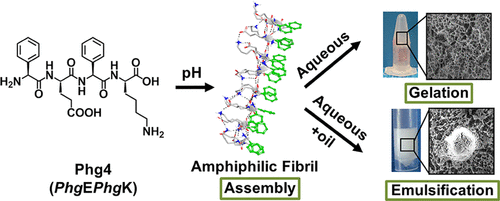
Understanding peptide self-assembly mechanisms and stability of the formed assemblies is crucial for the development of functional nanomaterials. Herein, we have adopted a rational design approach to demonstrate how a minimal structural modification to a nonassembling ultrashort ionic self-complementary tetrapeptide FEFK (Phe4) remarkably enhanced the stability of self-assembly into β-sheet nanofibers and induced hydrogelation. This was achieved by replacing flexible phenylalanine residue (F) by the rigid phenylglycine (Phg), resulting in a constrained analogue PhgEPhgK (Phg4), which positioned aromatic rings in an orientation favorable for aromatic stacking. Phg4 self-assembly into stable β-sheet ladders was facilitated by π-staking of aromatic side chains alongside hydrogen bonding between backbone amides along the nanofiber axis. The contribution of these noncovalent interactions in stabilizing self-assembly was predicted by in silico modeling using molecular dynamics simulations and semiempirical quantum mechanics calculations. In aqueous medium, Phg4 β-sheet nanofibers entangled at a critical gelation concentration ≥20 mg/mL forming a network of nanofibrous hydrogels. Phg4 also demonstrated a unique surface activity in the presence of immiscible oils and was superior to commercial emulsifiers in stabilizing oil-in-water (O/W) emulsions. This was attributed to interfacial adsorption of amphiphilic nanofibrils forming nanofibrilized microspheres. To our knowledge, Phg4 is the shortest ionic self-complementary peptide rationally designed to self-assemble into stable β-sheet nanofibers capable of gelation and emulsification. Our results suggest that ultrashort ionic-complementary constrained peptides or UICPs have significant potential for the development of cost-effective, sustainable, and multifunctional soft bionanomaterials.
中文翻译:

芳香堆积促进了超短离子互补肽序列的自组装:具有显着胶凝和界面特性的β-Sheet纳米纤维。
了解肽的自组装机制和形成的组装的稳定性对于功能纳米材料的开发至关重要。本文中,我们采用了合理的设计方法来证明对非组装超短离子自互补四肽F E F K(Phe4)的最小结构修饰如何显着增强自组装成β-片状纳米纤维和诱导水凝胶化的稳定性。这是通过用刚性苯基甘氨酸(Phg)代替柔性苯基丙氨酸残基(F)来实现的,其结果是受约束的类似物Phg E PhgK(Phg4),它将芳环定位在有利于芳族堆积的方向上。Phg4自组装成稳定的β-折叠梯形是通过芳族侧链的π-键化以及沿着纳米纤维轴的主链酰胺之间的氢键促进的。通过计算机模拟使用分子动力学模拟和半经验量子力学计算,预测了这些非共价相互作用对稳定自组装的贡献。在水性介质中,Phg4β-片状纳米纤维在临界胶凝浓度≥20mg / mL时缠结在一起,形成纳米纤维水凝胶网络。Phg4在不混溶的油存在下也表现出独特的表面活性,在稳定水包油(O / W)乳液方面优于商业乳化剂。这归因于两亲性纳米纤维形成纳米纤维化微球的界面吸附。据我们所知,Phg4是最短的离子自互补肽,经过合理设计,可以自组装为能够凝胶化和乳化的稳定的β-折叠纳米纤维。我们的研究结果表明,超短离子互补约束肽或UICP具有开发具有成本效益,可持续发展和多功能的软纳米材料的巨大潜力。
更新日期:2020-07-13
中文翻译:

芳香堆积促进了超短离子互补肽序列的自组装:具有显着胶凝和界面特性的β-Sheet纳米纤维。
了解肽的自组装机制和形成的组装的稳定性对于功能纳米材料的开发至关重要。本文中,我们采用了合理的设计方法来证明对非组装超短离子自互补四肽F E F K(Phe4)的最小结构修饰如何显着增强自组装成β-片状纳米纤维和诱导水凝胶化的稳定性。这是通过用刚性苯基甘氨酸(Phg)代替柔性苯基丙氨酸残基(F)来实现的,其结果是受约束的类似物Phg E PhgK(Phg4),它将芳环定位在有利于芳族堆积的方向上。Phg4自组装成稳定的β-折叠梯形是通过芳族侧链的π-键化以及沿着纳米纤维轴的主链酰胺之间的氢键促进的。通过计算机模拟使用分子动力学模拟和半经验量子力学计算,预测了这些非共价相互作用对稳定自组装的贡献。在水性介质中,Phg4β-片状纳米纤维在临界胶凝浓度≥20mg / mL时缠结在一起,形成纳米纤维水凝胶网络。Phg4在不混溶的油存在下也表现出独特的表面活性,在稳定水包油(O / W)乳液方面优于商业乳化剂。这归因于两亲性纳米纤维形成纳米纤维化微球的界面吸附。据我们所知,Phg4是最短的离子自互补肽,经过合理设计,可以自组装为能够凝胶化和乳化的稳定的β-折叠纳米纤维。我们的研究结果表明,超短离子互补约束肽或UICP具有开发具有成本效益,可持续发展和多功能的软纳米材料的巨大潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号