当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Plasma-Derived Extracellular Vesicle Phosphoproteomics through Chemical Affinity Purification.
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2020-05-12 , DOI: 10.1021/acs.jproteome.0c00151 Anton Iliuk 1, 2 , Xiaofeng Wu 3 , Li Li 2 , Jie Sun 4 , Marco Hadisurya 1 , Ronald S Boris 5 , W Andy Tao 1, 2, 3, 4, 6
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2020-05-12 , DOI: 10.1021/acs.jproteome.0c00151 Anton Iliuk 1, 2 , Xiaofeng Wu 3 , Li Li 2 , Jie Sun 4 , Marco Hadisurya 1 , Ronald S Boris 5 , W Andy Tao 1, 2, 3, 4, 6
Affiliation
|
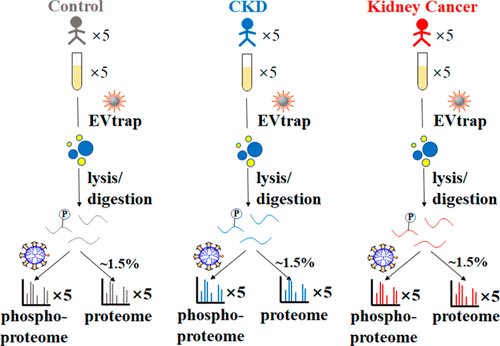
The invasive nature and the pain caused to patients inhibit the routine use of tissue biopsy-based procedures for cancer diagnosis and surveillance. The analysis of extracellular vesicles (EVs) from biofluids has recently gained significant traction in the liquid biopsy field. EVs offer an essential “snapshot” of their precursor cells in real time and contain an information-rich collection of nucleic acids, proteins, lipids, and so on. The analysis of protein phosphorylation, as a direct marker of cellular signaling and disease progression could be an important stepping stone to successful liquid biopsy applications. Here we introduce a rapid EV isolation method based on chemical affinity called EVtrap (extracellular vesicle total recovery and purification) for the EV phosphoproteomics analysis of human plasma. By incorporating EVtrap with high-performance mass spectrometry (MS), we were able to identify over 16 000 unique peptides representing 2238 unique EV proteins from just 5 μL of plasma sample, including most known EV markers, with substantially higher recovery levels compared with ultracentrifugation. Most importantly, more than 5500 unique phosphopeptides representing almost 1600 phosphoproteins in EVs were identified using only 1 mL of plasma. Finally, we carried out a quantitative EV phosphoproteomics analysis of plasma samples from patients diagnosed with chronic kidney disease or kidney cancer, identifying dozens of phosphoproteins capable of distinguishing disease states from healthy controls. The study demonstrates the potential feasibility of our robust analytical pipeline for cancer signaling monitoring by tracking plasma EV phosphorylation.
中文翻译:

通过化学亲和纯化的血浆衍生的细胞外囊泡磷酸蛋白质组学。
对患者造成的侵入性和疼痛抑制了基于组织活检的癌症诊断和监测程序的常规使用。对来自生物流体的细胞外囊泡 (EV) 的分析最近在液体活检领域获得了显着的关注。EV 实时提供其前体细胞的基本“快照”,并包含信息丰富的核酸、蛋白质、脂质等集合。作为细胞信号传导和疾病进展的直接标志物的蛋白质磷酸化分析可能是成功液体活检应用的重要垫脚石。在这里,我们介绍了一种基于化学亲和力的快速 EV 分离方法,称为 EVtrap(细胞外囊泡总回收和纯化),用于人血浆的 EV 磷酸蛋白质组学分析。通过将 EVtrap 与高性能质谱 (MS) 相结合,我们能够从仅 5 μL 血浆样品中鉴定出代表 2238 种独特 EV 蛋白的 16000 多种独特肽,包括大多数已知的 EV 标记物,与超速离心相比,回收率显着提高. 最重要的是,仅使用 1 mL 血浆就鉴定了代表 EV 中近 1600 种磷蛋白的 5500 多种独特的磷酸肽。最后,我们对诊断为慢性肾病或肾癌患者的血浆样本进行了定量 EV 磷酸蛋白质组学分析,确定了数十种能够区分疾病状态与健康对照的磷蛋白。
更新日期:2020-07-02
中文翻译:

通过化学亲和纯化的血浆衍生的细胞外囊泡磷酸蛋白质组学。
对患者造成的侵入性和疼痛抑制了基于组织活检的癌症诊断和监测程序的常规使用。对来自生物流体的细胞外囊泡 (EV) 的分析最近在液体活检领域获得了显着的关注。EV 实时提供其前体细胞的基本“快照”,并包含信息丰富的核酸、蛋白质、脂质等集合。作为细胞信号传导和疾病进展的直接标志物的蛋白质磷酸化分析可能是成功液体活检应用的重要垫脚石。在这里,我们介绍了一种基于化学亲和力的快速 EV 分离方法,称为 EVtrap(细胞外囊泡总回收和纯化),用于人血浆的 EV 磷酸蛋白质组学分析。通过将 EVtrap 与高性能质谱 (MS) 相结合,我们能够从仅 5 μL 血浆样品中鉴定出代表 2238 种独特 EV 蛋白的 16000 多种独特肽,包括大多数已知的 EV 标记物,与超速离心相比,回收率显着提高. 最重要的是,仅使用 1 mL 血浆就鉴定了代表 EV 中近 1600 种磷蛋白的 5500 多种独特的磷酸肽。最后,我们对诊断为慢性肾病或肾癌患者的血浆样本进行了定量 EV 磷酸蛋白质组学分析,确定了数十种能够区分疾病状态与健康对照的磷蛋白。


























 京公网安备 11010802027423号
京公网安备 11010802027423号