当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Temporal modulation of calcium sensing in hematopoietic stem cells is crucial for proper stem cell expansion and engraftment.
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2020-05-11 , DOI: 10.1002/jcp.29777 Merve Uslu 1, 2 , Esra Albayrak 1, 2 , Fatih Kocabaş 1, 2
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2020-05-11 , DOI: 10.1002/jcp.29777 Merve Uslu 1, 2 , Esra Albayrak 1, 2 , Fatih Kocabaş 1, 2
Affiliation
|
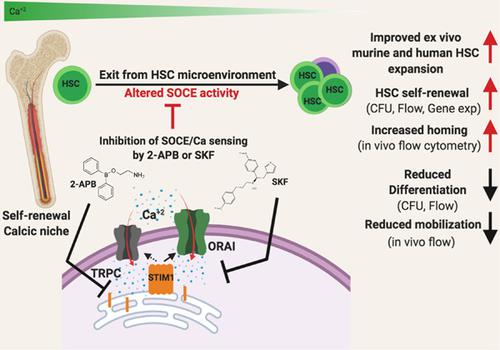
Hematopoietic stem cells (HSCs) are known to reside in a bone marrow (BM) niche, which is associated with relatively higher calcium content. HSCs sense and respond to calcium changes. However, how calcium‐sensing components modulate HSC function and expansion is largely unknown. We investigated temporal modulation of calcium sensing and Ca2+ homeostasis during ex vivo HSC culture and in vivo. Murine BM‐HSCs, human BM, and umbilical cord blood (UCB) mononuclear cells (MNCs) were treated with store‐operated calcium entry (SOCE) inhibitors SKF 96365 hydrochloride (abbreviated as SKF) and 2‐aminoethoxydiphenyl borate (2‐APB). Besides, K+ channel inhibitor TEA chloride (abbreviated as TEA) was used to compare the relationship between calcium‐activated potassium channel activities. Seven days of SKF treatment induced mouse and human ex vivo BM‐HSC expansion as well as UCB‐derived primitive HSC expansion. SKF treatment induced the surface expression of CaSR, CXCR4, and adhesion molecules on human hematopoietic stem and progenitor cells. HSCs expanded with SKF successfully differentiated into blood lineages in recipient animals and demonstrated a higher repopulation capability. Furthermore, modulation of SOCE in the BM‐induced HSC content and differentially altered niche‐related gene expression profile in vivo. Intriguingly, treatments with SOCE inhibitors SKF and 2‐APB boosted the mouse BM mesenchymal stem cell (MSC) and human adipose‐derived MSCs proliferation, whereas they did not affect the endothelial cell proliferation. These findings suggest that temporal modulation of calcium sensing is crucial in expansion and maintenance of murine HSCs, human HSCs, and mouse BM‐MSCs function.
中文翻译:

造血干细胞中钙感测的时间调节对于适当的干细胞扩增和植入至关重要。
已知造血干细胞(HSC)驻留在骨髓(BM)小生境中,这与相对较高的钙含量有关。HSC感知并响应钙的变化。但是,钙敏感成分如何调节HSC功能和扩展尚不清楚。我们调查了离体HSC培养和体内钙感应和Ca 2+稳态的时间调节。小鼠BM-HSC,人BM和脐带血(UCB)单个核细胞(MNC)用储库操作的钙进入(SOCE)抑制剂SKF 96365盐酸盐(缩写为SKF)和2-氨基乙氧基二苯基硼酸盐(2-APB)处理。此外,K +通道抑制剂TEA氯化物(简称为TEA)用于比较钙激活的钾通道活性之间的关系。SKF治疗的7天诱导了小鼠和人类离体BM-HSC的扩增以及UCB衍生的原始HSC的扩增。SKF处理可诱导CaSR,CXCR4和粘附分子在人造血干细胞和祖细胞上的表面表达。用SKF扩增的HSC成功地在受体动物中分化成血统,并表现出更高的再填充能力。此外,SOCE调节了BM诱导的HSC含量,并在体内差异性改变了与利基相关的基因表达谱。有趣的是,使用SOCE抑制剂SKF和2-APB可以促进小鼠BM间充质干细胞(MSC)和人脂肪来源的MSCs的增殖,而它们不影响内皮细胞的增殖。这些发现表明,钙感测的时间调节对于鼠HSC,人HSC和小鼠BM-MSC功能的扩展和维持至关重要。
更新日期:2020-05-11
中文翻译:

造血干细胞中钙感测的时间调节对于适当的干细胞扩增和植入至关重要。
已知造血干细胞(HSC)驻留在骨髓(BM)小生境中,这与相对较高的钙含量有关。HSC感知并响应钙的变化。但是,钙敏感成分如何调节HSC功能和扩展尚不清楚。我们调查了离体HSC培养和体内钙感应和Ca 2+稳态的时间调节。小鼠BM-HSC,人BM和脐带血(UCB)单个核细胞(MNC)用储库操作的钙进入(SOCE)抑制剂SKF 96365盐酸盐(缩写为SKF)和2-氨基乙氧基二苯基硼酸盐(2-APB)处理。此外,K +通道抑制剂TEA氯化物(简称为TEA)用于比较钙激活的钾通道活性之间的关系。SKF治疗的7天诱导了小鼠和人类离体BM-HSC的扩增以及UCB衍生的原始HSC的扩增。SKF处理可诱导CaSR,CXCR4和粘附分子在人造血干细胞和祖细胞上的表面表达。用SKF扩增的HSC成功地在受体动物中分化成血统,并表现出更高的再填充能力。此外,SOCE调节了BM诱导的HSC含量,并在体内差异性改变了与利基相关的基因表达谱。有趣的是,使用SOCE抑制剂SKF和2-APB可以促进小鼠BM间充质干细胞(MSC)和人脂肪来源的MSCs的增殖,而它们不影响内皮细胞的增殖。这些发现表明,钙感测的时间调节对于鼠HSC,人HSC和小鼠BM-MSC功能的扩展和维持至关重要。

























 京公网安备 11010802027423号
京公网安备 11010802027423号