Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2020-05-12 , DOI: 10.1016/j.bmc.2020.115555 Hongchuang Xu 1 , Minshu Wang 1 , Fengxu Wu 1 , Linsheng Zhuo 1 , Wei Huang 1 , Nengfang She 1
|
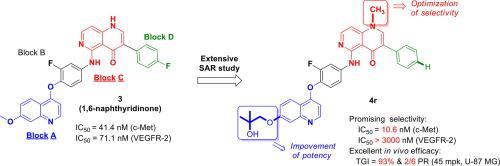
New N-substituted-3-phenyl-1,6-naphthyridinone derivatives are designed and synthesized, based on structural modification of our previously reported compound 3. Extensive enzyme-based SAR studies and PK evaluation led to the discovery of compound 4r, with comparable c-Met potency to that of Cabozantinib and high VEGFR-2 selectivity, while Cabozantinib displayed no VEGFR-2 selectivity. More importantly, at oral doses of 45 mg/kg (Q.D.), compound 4r exhibits significant tumor growth inhibition (93%) in a U-87MG human gliobastoma xenograft model. The promising selectivity against VEGFR-2 and excellent tumor growth inhibition of compound 4r suggest that it could be used as a new lead molecule for further discovery of selective type II c-Met inhibitors.
中文翻译:

发现带有喹啉部分的N-取代-3-苯基-1,6-萘啶酮衍生物作为针对VEGFR-2的选择性II型c-Met激酶抑制剂。
基于我们先前报道的化合物3的结构修饰,设计和合成了新的N-取代-3-苯基-1,6-萘啶酮衍生物。广泛的基于酶的SAR研究和PK评估导致发现了化合物4r,具有与Cabozantinib相当的c-Met效力和很高的VEGFR-2选择性,而Cabozantinib没有显示出VEGFR-2选择性。更重要的是,在45 mg / kg(QD)的口服剂量下,化合物4r在U-87MG人胶质母细胞瘤异种移植模型中显示出显着的肿瘤生长抑制作用(93%)。对VEGFR-2的有希望的选择性和对化合物4r的优异肿瘤生长抑制 提示它可用作进一步发现选择性II型c-Met抑制剂的新的先导分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号