当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Light-Switchable Yolk-Mesoporous Shell UCNPs@MgSiO3 for Nitric Oxide-Evoked Multidrug Resistance Reversal in Cancer Therapy.
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2020-05-12 , DOI: 10.1021/acsami.0c06102 Shihua Li 1 , Xiaorong Song 1 , Wei Zhu 1 , Yongling Chen 1 , Rong Zhu 1 , Liping Wang 1 , Xian Chen 1 , Jibin Song 1 , Huanghao Yang 1
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2020-05-12 , DOI: 10.1021/acsami.0c06102 Shihua Li 1 , Xiaorong Song 1 , Wei Zhu 1 , Yongling Chen 1 , Rong Zhu 1 , Liping Wang 1 , Xian Chen 1 , Jibin Song 1 , Huanghao Yang 1
Affiliation
|
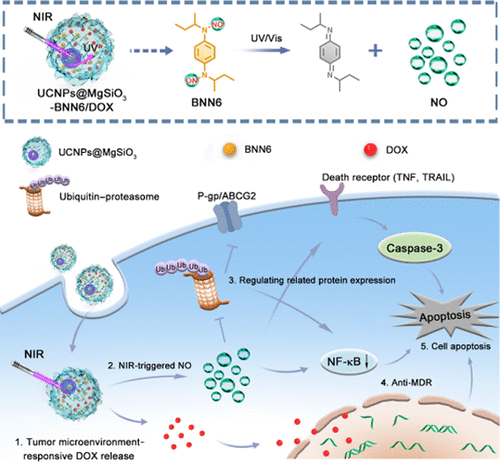
Gas therapy has emerged as a forceful strategy for augmenting the effects of chemotherapeutic drugs against cancer cells. However, it remains extremely challenging to effectively deliver gas into tissues of interest and unravel its underlying mechanisms. Herein, we designed a near-infrared (NIR) light-switchable nitric oxide (NO) delivery nanosystem for high-efficacy multidrug resistance (MDR) reversal in cancer therapy based on a yolk–shell upconverting nanoparticles@magnesium silica (UCNP@MgSiO3). The internal hollow cavity and flower-like mesoporous shell of UCNPs@MgSiO3 not only enabled a significantly high encapsulation capacity for the NO precursor (BNN6) and doxorubicin (DOX) but also allowed the enhanced cellular uptake, resulting in NIR-triggered NO generation and low pH-triggered DOX release in cancer cells. Mechanistically, intracellular NO can downregulate the drug efflux-related P-glycoprotein and adenosine 5′-triphosphate-binding cassette transporters, thereby increasing the DOX accumulation in the cell nuclei. Such combination therapy of NO and DOX induced the apoptosis of MDR cells and completely inhibited in vivo MDR tumor growth. We further elucidated the therapy mechanism via proteomic profiling, showcasing the downregulation of the ubiquitin–proteasome pathway and nuclear factor kappa-B signaling pathway in the NO-treated MDR cells. Therefore, our findings develop a promising nanoscale gas/drug delivery paradigm for fighting MDR tumors and providing molecular insights into cancer therapy.
中文翻译:

光开关卵黄介孔壳UCNPs @ MgSiO3用于一氧化氮诱发的癌症治疗中的多药耐药性逆转。
气体疗法已成为增强化疗药物对癌细胞作用的有效策略。然而,将气体有效地输送到感兴趣的组织并阐明其潜在机制仍然极具挑战性。本文中,我们设计了一种基于蛋黄-壳上转换纳米粒子@氧化镁二氧化硅(UCNP @ MgSiO 3)的近红外(NIR)光可转换一氧化氮(NO)输送纳米系统,用于癌症治疗中的高效多药耐药性(MDR)逆转。)。UCNPs @ MgSiO 3的内部空心腔和花状介孔壳不仅可以显着提高NO前体(BNN6)和阿霉素(DOX)的封装能力,还可以增强细胞摄取,从而导致NIR触发NO的产生和低pH触发DOX在癌细胞中的释放。从机制上讲,细胞内NO可以下调药物外排相关的P-糖蛋白和5'-三磷酸腺苷结合盒转运蛋白,从而增加DOX在细胞核中的积累。这种NO和DOX的联合疗法可诱导MDR细胞凋亡,并完全抑制体内MDR肿瘤的生长。我们通过蛋白质组学分析进一步阐明了治疗机制,显示了NO处理的MDR细胞中泛素-蛋白酶体途径和核因子κB信号通路的下调。因此,
更新日期:2020-05-12
中文翻译:

光开关卵黄介孔壳UCNPs @ MgSiO3用于一氧化氮诱发的癌症治疗中的多药耐药性逆转。
气体疗法已成为增强化疗药物对癌细胞作用的有效策略。然而,将气体有效地输送到感兴趣的组织并阐明其潜在机制仍然极具挑战性。本文中,我们设计了一种基于蛋黄-壳上转换纳米粒子@氧化镁二氧化硅(UCNP @ MgSiO 3)的近红外(NIR)光可转换一氧化氮(NO)输送纳米系统,用于癌症治疗中的高效多药耐药性(MDR)逆转。)。UCNPs @ MgSiO 3的内部空心腔和花状介孔壳不仅可以显着提高NO前体(BNN6)和阿霉素(DOX)的封装能力,还可以增强细胞摄取,从而导致NIR触发NO的产生和低pH触发DOX在癌细胞中的释放。从机制上讲,细胞内NO可以下调药物外排相关的P-糖蛋白和5'-三磷酸腺苷结合盒转运蛋白,从而增加DOX在细胞核中的积累。这种NO和DOX的联合疗法可诱导MDR细胞凋亡,并完全抑制体内MDR肿瘤的生长。我们通过蛋白质组学分析进一步阐明了治疗机制,显示了NO处理的MDR细胞中泛素-蛋白酶体途径和核因子κB信号通路的下调。因此,



























 京公网安备 11010802027423号
京公网安备 11010802027423号