当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective [4+2]-Cycloaddition with Chiral Alkenylboranes.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-05-11 , DOI: 10.1002/anie.202000652 Dongshun Ni 1 , Brittany P Witherspoon 2 , Hong Zhang 3, 4 , Chen Zhou 1 , K N Houk 3 , M Kevin Brown 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-05-11 , DOI: 10.1002/anie.202000652 Dongshun Ni 1 , Brittany P Witherspoon 2 , Hong Zhang 3, 4 , Chen Zhou 1 , K N Houk 3 , M Kevin Brown 1
Affiliation
|
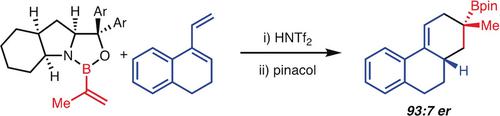
A method for the stereoselective [4+2]‐cycloaddition of alkenylboranes and dienes is presented. This transformation was accomplished through the introduction of a new strategy that involves the use of chiral N‐protonated alkenyl oxazaborolidines as dieneophiles. The reaction leads to the formation of products that can be readily derivatized to more complex structural motifs through stereospecific transformations of the C−B bond such as oxidation and homologation. Detailed computation evaluation of the reaction has uncovered a surprising role of the counterion on stereoselectivity.
中文翻译:

与手性烯基硼烷的立体选择性[4 + 2]-环加成。
提出了一种方法用于烯基硼烷和二烯的立体选择[4 + 2]-环加成反应。这种转化是通过引入一种新策略完成的,该策略涉及使用手性N质子化的烯基恶唑硼烷作为二烯亲和体。该反应导致形成产物,该产物可以通过C-B键的立体定向转化(例如氧化和同源化)而容易地衍生为更复杂的结构图案。反应的详细计算评估发现反离子对立体选择性的惊人作用。
更新日期:2020-07-01
中文翻译:

与手性烯基硼烷的立体选择性[4 + 2]-环加成。
提出了一种方法用于烯基硼烷和二烯的立体选择[4 + 2]-环加成反应。这种转化是通过引入一种新策略完成的,该策略涉及使用手性N质子化的烯基恶唑硼烷作为二烯亲和体。该反应导致形成产物,该产物可以通过C-B键的立体定向转化(例如氧化和同源化)而容易地衍生为更复杂的结构图案。反应的详细计算评估发现反离子对立体选择性的惊人作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号