当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The binding of the APT1 domains to phosphoinositides is regulated by metal ions in vitro.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-05-11 , DOI: 10.1016/j.bbamem.2020.183349 Damian Kolakowski 1 , Joanna Kaminska 1 , Teresa Zoladek 1
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-05-11 , DOI: 10.1016/j.bbamem.2020.183349 Damian Kolakowski 1 , Joanna Kaminska 1 , Teresa Zoladek 1
Affiliation
|
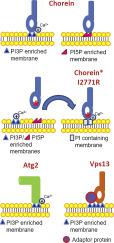
Chorein is a protein of the Vps13 family, and defects in this protein cause the rare neurodegenerative disorder chorea-acanthocytosis (ChAc). Chorein is involved in the actin cytoskeleton organization, calcium ion flux, neuronal cell excitability, exocytosis and autophagy. The function of this protein is poorly understood, and obtaining this knowledge is a key to finding a cure for ChAc. Chorein, as well as the Vps13 protein from yeast, contains the APT1 domain. Our previous research has shown that the APT1 domain from yeast Vps13 (yAPT1v) binds phosphatidylinositol 3-phosphate (PI3P) in vitro. In this study, we showed that although the APT1 domain from chorein (hAPT1) binds to PI3P it could not functionally replace yAPT1v. The hAPT1 domain binds, in addition to PI3P, to phosphatidylinositol 5-phosphate (PI5P). The binding of hAPT1 to PI3P, unlike the binding of yAPT1v to PI3P, is regulated by the bivalent ions calcium and magnesium. Regulation of PI3P binding via calcium is also observed for the APT1 domain of yeast autophagy protein Atg2. The substitution I2771R, found in chorein of patient suffering from ChAc, reduces the binding of the hAPT1 domain to PI3P and PI5P. These results suggest that the ability of APT1 domains to bind phosphoinositides is regulated differently in yeast and human protein and that this regulation is important for chorein function.
中文翻译:

APT1结构域与磷酸肌醇的结合在体外受金属离子调控。
软骨素是Vps13家族的一种蛋白质,该蛋白质中的缺陷会引起罕见的神经退行性疾病舞蹈-棘皮细胞增多症(ChAc)。Chorein参与肌动蛋白的细胞骨架组织,钙离子通量,神经元细胞兴奋性,胞吐作用和自噬。对该蛋白质的功能了解甚少,获得该知识是寻找ChAc治愈方法的关键。软骨素以及来自酵母的Vps13蛋白均包含APT1结构域。我们以前的研究表明,酵母Vps13(yAPT1v)的APT1域在体外与磷脂酰肌醇3-磷酸(PI3P)结合。在这项研究中,我们表明,尽管来自于chorein的APT1域(hAPT1)与PI3P结合,但是它不能功能性地取代yAPT1v。除PI3P外,hAPT1结构域还与磷脂酰肌醇5-磷酸(PI5P)结合。hAPT1与PI3P的结合,与yAPT1v与PI3P的结合不同,它受二价离子钙和镁的调节。酵母自噬蛋白Atg2的APT1结构域也观察到通过钙调节PI3P结合。在患有ChAc的患者的胰蛋白酶中发现的取代I2771R降低了hAPT1域与PI3P和PI5P的结合。这些结果表明,APT1结构域结合磷酸肌醇的能力在酵母和人类蛋白质中受到不同的调节,并且这种调节对于胰蛋白酶的功能很重要。
更新日期:2020-05-11
中文翻译:

APT1结构域与磷酸肌醇的结合在体外受金属离子调控。
软骨素是Vps13家族的一种蛋白质,该蛋白质中的缺陷会引起罕见的神经退行性疾病舞蹈-棘皮细胞增多症(ChAc)。Chorein参与肌动蛋白的细胞骨架组织,钙离子通量,神经元细胞兴奋性,胞吐作用和自噬。对该蛋白质的功能了解甚少,获得该知识是寻找ChAc治愈方法的关键。软骨素以及来自酵母的Vps13蛋白均包含APT1结构域。我们以前的研究表明,酵母Vps13(yAPT1v)的APT1域在体外与磷脂酰肌醇3-磷酸(PI3P)结合。在这项研究中,我们表明,尽管来自于chorein的APT1域(hAPT1)与PI3P结合,但是它不能功能性地取代yAPT1v。除PI3P外,hAPT1结构域还与磷脂酰肌醇5-磷酸(PI5P)结合。hAPT1与PI3P的结合,与yAPT1v与PI3P的结合不同,它受二价离子钙和镁的调节。酵母自噬蛋白Atg2的APT1结构域也观察到通过钙调节PI3P结合。在患有ChAc的患者的胰蛋白酶中发现的取代I2771R降低了hAPT1域与PI3P和PI5P的结合。这些结果表明,APT1结构域结合磷酸肌醇的能力在酵母和人类蛋白质中受到不同的调节,并且这种调节对于胰蛋白酶的功能很重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号