当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NHC-catalyzed β-specific addition of N-based nucleophiles to allenoates
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-05-11 , DOI: 10.1039/d0qo00189a Wenchao Wu 1, 2, 3, 4, 5 , Shuangli Xu 1, 2, 3, 4, 5 , Yan Zhang 1, 2, 3, 4, 5 , Xiu Wang 1, 2, 3, 4, 5 , Ruotong Li 1, 2, 3, 4, 5 , Fang Sun 1, 2, 3, 4, 5 , Chenxia Yu 1, 2, 3, 4, 5 , Tuanjie Li 1, 2, 3, 4, 5 , Donghui Wei 5, 6, 7, 8 , Changsheng Yao 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-05-11 , DOI: 10.1039/d0qo00189a Wenchao Wu 1, 2, 3, 4, 5 , Shuangli Xu 1, 2, 3, 4, 5 , Yan Zhang 1, 2, 3, 4, 5 , Xiu Wang 1, 2, 3, 4, 5 , Ruotong Li 1, 2, 3, 4, 5 , Fang Sun 1, 2, 3, 4, 5 , Chenxia Yu 1, 2, 3, 4, 5 , Tuanjie Li 1, 2, 3, 4, 5 , Donghui Wei 5, 6, 7, 8 , Changsheng Yao 1, 2, 3, 4, 5
Affiliation
|
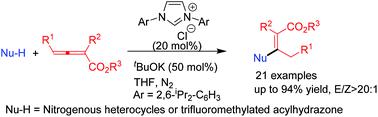
N-Heterocyclic carbene (NHC) catalyzed regiospecific β-additions of nitrogen-containing nucleophiles including nitrogenous heterocycles and trifluoromethylated acylhydrazone to allenoates were accomplished under mild reaction conditions, harnessing NHC as a Lewis base to furnish branched N-alkyl compounds in moderate to good yields with high E/Z selectivity and broad substrate scope. DFT calculations on the possible reaction mechanisms show that the β-addition pathway is more energetically favourable than the γ-addition pathway, which is in agreement with the experimental observations.
中文翻译:

NHC催化的N基亲核试剂的β特异性加成物
在温和的反应条件下,完成了N-杂环卡宾(NHC)催化的含氮亲核试剂(包括含氮杂环和三氟甲基化酰hydr)的区域特异性β加成反应,从而利用NHC作为Lewis碱,以中等至良好的收率提供了支链N-烷基化合物具有较高的E / Z选择性和广泛的底物范围。对可能的反应机理的DFT计算表明,β-添加途径比γ-添加途径在能量上更有利,这与实验观察结果一致。
更新日期:2020-06-30
中文翻译:

NHC催化的N基亲核试剂的β特异性加成物
在温和的反应条件下,完成了N-杂环卡宾(NHC)催化的含氮亲核试剂(包括含氮杂环和三氟甲基化酰hydr)的区域特异性β加成反应,从而利用NHC作为Lewis碱,以中等至良好的收率提供了支链N-烷基化合物具有较高的E / Z选择性和广泛的底物范围。对可能的反应机理的DFT计算表明,β-添加途径比γ-添加途径在能量上更有利,这与实验观察结果一致。


























 京公网安备 11010802027423号
京公网安备 11010802027423号